Abstract
Aim
To investigate long-term effects of sodium-glucose co-transporter 2 inhibitor (SGLT2i) on anthropometric and metabolic factors in Japanese patients with type 2 diabetes (T2DM).
Patients and Methods
This is a retrospective observation study. Forty-six outpatients with T2DM (32 men and 14 women, 51 ± 13 years old, BMI 27.9 ± 4.8, means ± S.D.) who had been treated by SGLT2i for 2 years were selected and their metabolic and anthropometric data were retrieved from medical records retrospectively. Regular instruction for diet and exercise had been performed throughout the administration of SGLT2i in outpatient clinic basis.
Results
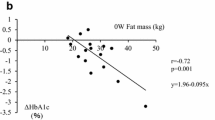
By the administration of SGLT2i for 2 years, body weight and body fat amount were significantly reduced (P < 0.0001) in spite of no change in skeletal muscle mass. HbA1c (P < 0.0001), liver function and lipid profile (P < 0.01) were ameliorated and eGFR was reduced significantly (P < 0.0001). It is of note that the reduction of body weight was strongly correlated to that of body fat (r = 0.951, P < 0.0001) with no correlation to the change of skeletal muscle mass. The reduction of HbA1c was strongly correlated to the baseline HbA1c (r = − 0.922, P < 0.0001) and modestly correlated to the baseline eGFR (r = − 0.449, P < 0.01). Multivariate analysis revealed a weak relationship between the amelioration of HbA1c and the reduction of body weight.
Conclusion
SGLT2i can effectively reduce body weight and body fat mass independent of the blood glucose improvement or the renal function. Under the periodical instruction for nutrition and exercise this oral hypoglycemic agent can be safely administered for a long term without a risk for sarcopenia.


Similar content being viewed by others
References
Japan Diabetes Clinical Data Management Study Group. http://jddm.jp/data/index-2019/.
Tajiri Y, Takei R, Mimura K, et al. Attenuated metabolic effect of waist measurement in Japanese female patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;82:66–72.
Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res. 2007;75:690–701.
Welch GW, Jacobson AM, Polonsky WH. The problem areas in diabetes scale. An evaluation of its clinical utility. Diabetes Care. 1997;20:760–6.
Guideline Committee of the Japan Diabetes Society. Treatment guide for diabetes 2013. Japan Diabetes Society.
Hu H, Hori A, Nishiura C, et al. Hba1c, blood pressure, and lipid control in people with diabetes: Japan Epidemiology Collaboration on Occupational Health Study. PLoS ONE. 2016;11:e0159071.
Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–31.
Ferrannini G, Hach T, Crowe S, et al. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730–5.
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Ohta A, Kato H, Ishii S, et al. Ipragliflozin, a sodium glucose co-transporter 2 inhibitor, reduces intrahepatic lipid content and abdominal visceral fat volume in patients with type 2 diabetes. Expert Opin Pharmacother. 2017;18:1433–8.
Bianchi L, Volpato S. Muscle dysfunction in type 2 diabetes: a major threat to patient’s mobility and independence. Acta Diabetol. 2016;53:879–89.
Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obes Surg. 2006;16:1602–8.
Gomez-Arbelaez D, Bellido D, Castro AI, et al. Body composition changes after very-low-calorie ketogenic diet in obesity evaluated by 3 standardized methods. J Clin Endocrinol Metab. 2017;102:488–98.
Jendle J, Nauck MA, Matthews DR, et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab. 2009;11:1163–72.
Inoue H, Morino K, Ugi S, et al. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: a randomized clinical trial. J Diabetes Investig. 2019;10:1012–21.
Sugiyama S, Jinnouchi H, Kurinami N, et al. Dapagliflozin reduces fat mass without affecting muscle mass in type 2 diabetes. J Atheroscler Thromb. 2018;25:467–76.
Nakamura I, Maegawa H, Tobe K, et al. Safety and effectiveness of Ipragliflozin for type 2 diabetes in Japan: 12-month interim results of the STELLA-LONG TERM post-marketing surveillance study. Adv Ther. 2019;36:923–49.
Leiter LA, Forst T, Polidori D, et al. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42:25–32.
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–7.
Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–71.
Ikeda S, Takano Y, Schwab D, et al. Effect of renal impairment on the pharmacokinetics and pharmacodynamics of tofogliflozin (A SELECTIVE SGLT2 inhibitor) in patients with type 2 diabetes mellitus. Drug Res (Stuttg). 2019;69:314–22.
Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–73.
Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–84.
Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–60.
Haneda M, Seino Y, Inagaki N, et al. Influence of renal function on the 52-week efficacy and safety of the sodium glucose cotransporter 2 inhibitor Luseogliflozin in Japanese patients with type 2 diabetes mellitus. Clin Ther. 2016;38:66–88 (e20).
Obata A, Kubota N, Kubota T, et al. Tofogliflozin improves insulin resistance in skeletal muscle and accelerates lipolysis in adipose tissue in male mice. Endocrinology. 2016;157:1029–42.
Xu L, Nagata N, Nagashimada M, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–49.
Sha S, Polidori D, Heise T, et al. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16:1087–95.
Almeida JFQ, Shults N, de Souza AMA, et al. Short-term very low caloric intake causes endothelial dysfunction and increased susceptibility to cardiac arrhythmias and pathology in male rats. Exp Physiol. 2020;105:1172–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sakamoto, M., Goto, Y., Nagayama, A. et al. Two-year administration of sodium-glucose co-transporter 2 inhibitor brought about marked reduction of body fat independent of skeletal muscle amount or glycemic improvement in Japanese patients with type 2 diabetes. Diabetol Int 13, 117–123 (2022). https://doi.org/10.1007/s13340-021-00512-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-021-00512-7