Abstract
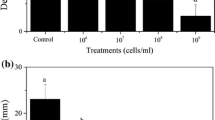
One isolate of Colletotrichum truncatum was found recently causing severe anthracnose symptoms and leading to the death of beggartick (Bidens pilosa L. and Bidens subalternans DC.), one of the major weeds of Brazilian agriculture. This isolate, namely UFU 280, was selected for development of a mycoherbicide against this weed. Associated of beggartick, one of the, Here, results of a preliminary attempt to develop a protocol for mass production of inoculum (conidia) and fungal biomass of C. truncatum was performed aimed at paving the way for greenhouse and field evaluations of this biocontrol candidate. Isolates of Colletotrichum spp. have been successfully produced in the past to serve as the active ingredients of mycoherbicides. The method of choice has been the production of propagules through liquid fermentation. We assessed the effect of several options of liquid media recipes, type of seeding of medium, pH levels, incubation lengths, incubation temperatures and agitation speeds on the shaking speed on the concentration of conidia obtained per volume of medium. Additionally, a possible effect of the kind of medium utilized over the virulence of the inoculum was also evaluated through an inoculation study. We found that an adequate amount of conidia of C. truncatum (isolate - UFU 280) can be obtained in ME liquid culture medium, adjusted to a pH of 9.0, seeded with a conidial suspension and incubated for 6 days, under a regime of orbital shaking of 150 rpm, at temperatures ranging from 20 to 25 °C. Mortality of beggartick plants using conidia produced in different liquid culture media was of 100%.

Similar content being viewed by others
References
Auld BA (1998) On the social value of biological control of weeds. Int J Soc Econ 25(6/7/8):1199–1206
Bailey JA, Jeger MJ (1992) Colletotrichum: biology, pathology and control. CAB International, Kew
Bailey BA, Hebbar KP, Neil NRO, Lewis JA (2004) Production of Pleospora papaveracea biomass in liquid culture and its infectivity on opium poppy (Papaver somniferum). Weed Sci 52:91–97
Baio FHR, Pires LF, Tomquelski G (2013) Mapeamento de picão preto resistente aos herbicidas inibidores da ALS na região sul Mato-grossense. Biosci J 29(1):59–64
Barran LR, Schneider EF, Seaman WL (1977) Requirements for the rapid conversion of macroconidia of Fusarium sulphureum to chlamydospores. Can J Microbiol 23:148–151
Barreto RW (2009) Controle biológico de plantas daninhas com fitopatógenos. In: Bettiol W, Morandi MAB (eds) Biocontrole de doenças de planta: uso e perspectivas. Jaguariúna, Embrapa Meio Ambiente, pp 101–128
Barreto RW, Elisson CA, Sier MK, Evans HC (2012) Biological control of weeds with plant pathogens: four decades on. In: Abrol D, Kashmir S (eds.) Integrated pest management. CAB International, University of Agricultural Sciences and Technology, Jammu, pp. 299-350
Bowers RC (1982) Commercialization of microbial biological control agents. In: Charudattan R, Walker HL (eds) Biological control of weeds with plant pathogens, 2nd edn. John Wiley and Sons, New York, pp 157–173
Boyette CD, Quimby Júnior PC, Connick Júnior WJ, Daigle DJ, Fulgham FE (1991) Progress in the production, formulation, and application of mycoherbicides. In: Tebeest DO (ed) Microbial control of weeds, 3rd edn. Chapman and Hall, New York, pp 209–222
Boyette CD, Hamed KA, Johnson B, Hoagland RE, Weaver MA (2014) Biological control of the weed Sesbania exaltata using a microsclerotia formulation of the bioherbicide Colletotrichum truncatum. Am J Plant Sci 5:2672–2685
Butt TM, Jackson CW, Murugan W (2001) Fungi as biocontrol agents, progress, problems and potentials. CBBS Publshing Co, London, pp 240–242
Calam CT (1986) Shake-flask fermentation. In: Arnold LD, Nadine AS (eds.) Manual of industrial microbiology and biotechnology. American Society for Microbiology, Washington, DC, pp 59–63
Charudattan R (2001) Biological control of weeds by means of plant pathogens: significance for integrated weed, management in modern agro-ecology. BioControl 46:229–260
Churchill BW (1982) Mass production of microorganisms for biological control. In: Charudattan R, Walker HL (eds) Biological control of weeds with plant pathogens. John Willey & Sons, New York, pp 139–156
Cliquet S, Ash G, Cother E (2004) Production of chlamydospores and conidia in submerged culture by Rhynchosporium alismatis, a mycoherbicide of Alismataceae in rice crops. Biocontrol Sci Tech 14:801–810
Deacon J (2006) Fungal biology. Institute of Cell and Molecular Biology, 4th edn. Blackwell Publishing Ltd. University of Edinburgh, Edinburgh 380 p
Dhingra OD, Sinclair JB (1995) Basic methods in plant pathology, 2nd edn. Lewis Publishers, CRC Press, Boca Raton 434p
Diarra C, Ciotola M, Hallet SG, Hess DE, Watson AK (1996) Mass production of Fusarium oxysporum (M12-4ª), a biocontrol agent for Striga hermonthica. Proceedings of the IX International symposium on biological control of weeds; 1996 January 19-26; Stellenbosch. South África. University of Cape Town. pp 149-152
Fargues J, Smits N, Vidal C, Vey A, Vega F, Mercadier G, Quimby P (2001) Effect of liquid culture media on morphology, growth, propagule production, and pathogenic activity of the Hyphomycete, Metarhizium flavoviride. Mycopathologia 154:127–137
French ER, Nielsen LW (1966) Production of macroconidia of Fusarium oxysporum fsp. batatas and their conversion to chlamydospores. Phytopathology 56:1322
Gardner K, Wiebe MG, Gillespie AT, Trinci APJ (2000) Production of chlamydospores of the nematode-trapping Duddingtonia flagrans in shake flask culture. Mycol Res 104:205–209
Garraway MO, Evans RC (1984) Fungal nutrition and physiology. Wiley, New York
Gelmini GA (2001) Resistência de biótipos de Euphorbia heterophylla L., Bidens subalternans L. e Brachiaria plantaginea (Link) Hitchc. a herbicidas utilizados na cultura da soja (Glycine max (L.) Merril). Dissertação (Mestrado em Fitotecnia), Escola Superior de Agricultura “Luiz de Queiroz”, Piracicaba
Ghosheh HZ (2005) Constraints in implementing biological weed control: a review. Weed-Biol-Manag 5(3):83–92
Griffin D (1994) Fungal Physiology, 2nd edn. Wiley-Liss, New York
Guatimosim E, Pinto HJ, Pereira OL, Fuga CAG, Vieira BS, Barreto RW (2015) Pathogenic mycobiota of the weeds Bidens pilosa and Bidens subalternans. Trop Plant Pathol 40:64–83
Hallet SG (2005) Where are the bioherbicides? Weed-Science 53(3):404–415
Holm L (1991) The world’s worst weeds – distribution and biology. Krieger Publishing Company, Malabar
Hrac-BR - Associação brasileira de ação a resistência de plantas aos herbicidas. (2017) http://www.hrac-br.com.br/. Accessed 10 Oct 2017
Jackson MA, Schisler DA (1995) Liquid culture production of microsclerotia of Colletotrichum truncatum for use as bioherbicidal propagules. Mycol Res 99(7):879–884
Jackson MA, Schisler DA (2002) Selecting fungal biocontrol agents amenable to production by liquid culture fermentation. Proceedings of the 7th meeting of the IOBC OILB; 2002; Kusadasi. Turkey IOBC/WPRS bulletin. pp 387-390
Kissmann KG, Groth D (1999) Plantas infestantes e nocivas. Tomo II, São Paulo: BASF Brasileira. 978 p
McQuilken MP, Whipps JM, Cooke RC (1990) Oospores of the biocontrol agent Phytium oligandrum bulk-produced in liquid culture. Mycol Res 94:613–616. https://doi.org/10.1016/S0953-7562(09)80661-8
Moraes C (2009) Produção massal e influência de fatores físicos no cultivo e viabilidade de Bipolaris euphorbiae. Dissertação (Mestrado em Microbiologia Agropecuária), Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista, Jaboticabal
Pereira JM, Barreto RW, Ellison C, Maffia LA (2003) Corynespora cassiicola f. sp. lantanae: a potential biocontrol agent for Lantana camara from Brazil. Biol Control 26:21–31
R Development Core Team (2011) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2011. Available in <http://R-project.org>
Rizzardi MA, Vidal RA, Fleck NG, Agostinetto D (2002) Resistência de plantas aos herbicidas inibidores da acetolactato sintase. Planta Daninha 20(1):149–158
Schisler DA, Jackson MA, Bothast RJ (1991) Influence of nutrition during conidiation of Colletotrichum truncatum on conidial germination and efficacy in inciting disease in Sesbania exaltata. Phytopathology 81:587–590
Shearer JF, Jackson MA (2006) Liquid culturing of Microsclerotia of Mycoleptodiscus terrestris, a potential biological control agent for the management of Hydrilla. Biol Control 38:298–306
Silman RW, Nelsen TC, Bothast RJ (1991) Comparison of culture methods for production of Colletotrichum truncatum spores for use as a mycoherbicide. FEMS Microbiol Lett 79:69–74
Stowell LJ (1991) Submerged fermentation of biological herbicides. In: TeBeest DO (ed) Microbial control of weeds, 3rd edn. Chapman and Hall, New York, pp 225–261
Tan WZ, Li QJ, Qing L (2002) Biological control of alligatorweed (Alternanthera philoxeroides) with a Fusarium sp. BioControl 47:463–479
Van Winkelhoff AJ, Mccoy CW (1984) Conidiation of Hirsutella thompsonii var. synnematosa in submerged culture. J Invertebr Pathol 43:59–68
Vieira BS, Barreto RW (2010) Liquid culture production of chlamydospores of Lewia chlamidosporiformans (Ascomycota: Pleosporales), a mycoherbicide candidate for wild poinsettia. Australas Plant Pathol 39:154–160
Yandoc-Ables CB, Rosskopf EN, Charudattan R (2006a) Plant pathogens at work: progress and possibilities for weed biocontrol. Part 1: classical vs. bioherbicidal approach http://www.apsnet.org/publications/apsnetfeatures/Pages/WeedBiocontrolPart1.aspx
Yandoc-Ables CB, Rosskopf EN, Charudattan R (2006b) Plant pathogens at work: progress and possibilities for weed biocontrol. Part 2: improving weed control efficiency http://www.apsnet.org/publications/apsnetfeatures/Pages/WeedBiocontrolPart2.aspx
Zauza EAV, Alfenas AC, Mafia GR (2007) Esterilização, preparo de meios de cultura e fatores associados ao cultivo de fitopatógenos. In: Alfenas CA, Mafia RG (eds) Métodos em Fitopatologia. UFV, Viçosa, p 42
Zhang W, Sulz M, Bailey KL (2001) Growth and spore production of Plectosporium tabacinum. Can J Bot 79:1297–1306
Zhao S, Shamoun SF (2006) Effects of culture media, temperature, pH, and light on growth, sporulation, germination, and bioherbicidal efficacy of Phoma exigua, a potential biological control agent for salal (Gaultheria shallon). Biocontrol Sci Tech 16(10):1043–1055
Acknowledgements
The authors wish to acknowledge The Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vieira, B.S., Dias, L.V.S.A., Langoni, V.D. et al. Liquid fermentation of Colletotrichum truncatum UFU 280, a potential mycoherbicide for beggartick. Australasian Plant Pathol. 47, 277–283 (2018). https://doi.org/10.1007/s13313-018-0555-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-018-0555-y