Abstract
Trastuzumab is by far the drug of choice for treatment of human epidermal growth factor receptor 2 (Her2) overexpressing breast cancer patients. However, frequently, the therapy remains ineffective due to the induced drug resistance. In spite of various reported mechanisms, we hypothesize that the acquired resistance to trastuzumab might be attributed to the failure of the drug to activate phosphatase and tensin homolog (PTEN) mainly due to the high level of reduced thioredoxin-1 protein among the resistant cells. In the present study, the effect(s) of PX-12, a Trx-1 inhibitor, was examined on proliferation of breast cancer cells which are unresponsive to trastuzumab. Treatment of the cells with PX-12 (5 μM) and trastuzumab (10 μg/ml) reduced cells viabilities, p-Akt, and Bcl2 levels while increasing the levels of reactive oxygen species (ROS) and p-JNK with consequent higher levels of G1 arrest and apoptosis among the resistant cells compared to parental trastuzumab sensitive cells. The most significant observation was that PX-12/trastuzumab co-treatment enhanced the cell membrane localization of PTEN which is believed to be the active biological form of the signal. Our data confirmed that Trx-1 inhibition is required for chemosensitization of resistant breast cancer cells to anti-Her2 therapy, and this approach might offer an alternative clinical strategy for preventing acquired resistance.









Similar content being viewed by others
References
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CEJR, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26.
Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–9.
De Laurentiis M, Cancello G, Zinno L, Montagna E, Malorni L, Esposito A, et al. Targeting HER2 as a therapeutic strategy for breast cancer: a paradigmatic shift of drug development in oncology. Ann Oncol. 2005;16:iv7–13.
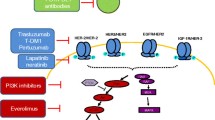
Lan KH, Lu C, Yu D. Mechanisms of trastuzumab resistance and their clinical implications. Ann N Y Acad Sci. 2005;1059:70–5.
Piccart M. Circumventing de novo and acquired resistance to trastuzumab: new hope for the care of ErbB2-positive breast cancer. Clin Breast Cancer. 2008;8:S100–13.
Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–28.
Nahta R, Takahashi T, Ueno NT, Hung MC, Esteva FJ. P27kip1 down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res. 2004;64:3981–6.
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–27.
Parsons R, Simpson L. PTEN and cancer. Methods Mol Biol. 2003;222:147–66.
Takei Y, Saga Y, Mizukami H, Takayama T, Ohwada M, Ozawa K, et al. Overexpression of PTEN in ovarian cancer cells suppresses ip dissemination and extends survival in mice. Mol Cancer Ther. 2008;7:704–11.
Squire JA. TMPRSS2-ERG and PTEN loss in prostate cancer. Nat Genet. 2009;41:509–10.
Seront E, Pinto A, Bouzin C, Bertrand L, Machiels JP, Feron O. PTEN deficiency is associated with reduced sensitivity to mTOR inhibitor in human bladder cancer through the unhampered feedback loop driving PI3K/Akt activation. Br J Cancer. 2013;109:1586–92.
Lin PY, Fosmire SP, Park SH, Park JY, Baksh S, Modiano JF, et al. Attenuation of PTEN increases p21 stability and cytosolic localization in kidney cancer cells: a potential mechanism of apoptosis resistance. Mol Cancer. 2007;6:16.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
Yang Y, Shao N, Luo G, Li L, Zheng L, Nilsson-Ehle P, et al. Mutations of PTEN gene in gliomas correlate to tumor differentiation and short-term survival rate. Anticancer Res. 2010;30:981–5.
Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–22.
Liu KJL, Yin B, Zhang R, Zhang J, Li P, et al. Distinct roles for PTEN in prevention of T cell lymphoma and autoimmunity in mice. J Clin Invest. 2010;120:2497–507.
Fulcher L, Friedrichs W, Grünwald V, Ray R, Hidalgo M. Reduced PTEN expression in breast cancer cells confers susceptibility to inhibitors of the PI3 kinase/Akt pathway. Ann Oncol. 2004;15:1510–6.
Gu J, Tamura M, Pankov R, Danen EH, Takino T, Matsumoto K, et al. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J Cell Biol. 1999;146:389–404.
Georgescu MM. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1:1170–7.
Meuillet EJ, Mahadevan D, Berggren M, Coon A, Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN’s lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN’s tumor suppressor activity. Arch Biochem Biophys. 2004;429:123–33.
Das KC, Das CK. Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: redox independent functions. Biochem Biophys Res Commun. 2000;277:443–7.
Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol. 2007;7(4):392–7.
Wangpaichitr M, Sullivan EJ, Theodoropoulos G, Wu C, You M, Feun LG, et al. The relationship of thioredoxin-1 and cisplatin resistance: its impact on ROS and oxidative metabolism in lung cancer cells. Mol Cancer Ther. 2012;11:604–15.
Kim SJ, Miyoshi Y, Taguchi T, Tamaki Y, Nakamura H, Yodoi J, et al. High thioredoxin expression is associated with resistance to docetaxel in primary breast cancer. Clin Cancer Res. 2005;11:8425–30.
Lechner S, Müller-Ladner U, Neumann E, Spöttl T, Schlottmann K, Rüschoff J, et al. Thioredoxin reductase 1 expression in colon cancer: discrepancy between in vitro and in vivo findings. Lab Investig. 2003;83:1321–31.
Noda N, Ochiai A, Miyazaki K, Sugimura T, Terada M, Wakasugi H. Detection of thioredoxin in gastric cancer: association with histological type. Antioxid Redox Signal. 2000;2:519–28.
Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-Imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1α and vascular endothelial growth factor formation 1. Mol Cancer Ther. 2003;2:235–43.
Tonissen KF, Di Trapani G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol Nutr Food Res. 2009;53:87–103.
Lebel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–31.
Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WA. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol. 2005;25:6211–24.
Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD, Maihle NJ. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res. 2009;69:2191–4.
Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–90.
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402.
Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–72.
Ravi D, Muniyappa H, Das KC. Endogenous thioredoxin is required for redox cycling of anthracyclines and p53-dependent apoptosis in cancer cells. J Biol Chem. 2005;280:40084–96.
Bloomfield KL, Osborne SA, Kennedy DD, Clarke FM, Tonissen KF. Thioredoxin-mediated redox control of the transcription factor Sp1 and regulation of the thioredoxin gene promoter. Gene. 2003;319:107–16.
Sakurai A, Yuasa K, Shoji Y, Himeno S, Tsujimoto M, Kunimoto M, et al. Overexpression of thioredoxin reductase 1 regulates NF‐κB activation. J Cell Physiol. 2004;198:22–30.
Wei SJ, Botero A, Hirota K, Bradbury CM, Markovina S, Laszlo A, et al. Thioredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer Res. 2000;60:6688–95.
Naranjo-Suarez S, Carlson BA, Tobe R, Yoo MH, Tsuji PA, Gladyshev VN, et al. Regulation of HIF-1α activity by overexpression of thioredoxin is independent of thioredoxin reductase status. Mol Cells. 2013;36:151–7.
Manoharan R, Seong HA, Ha H. Thioredoxin inhibits MPK38-induced ASK1, TGF‐β, and p53 function in a phosphorylation-dependent manner. Free Radic Biol Med. 2013;63:313–24.
Schwertassek U, Haque A, Krishnan N, Greiner R, Weingarten L, et al. Reactivation of oxidized PTP1B and PTEN by thioredoxin 1. FEBS J. 2014;281:3545–58.
Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–42.
Kim YH, Coon A, Baker AF, Powis G. Antitumor agent PX-12 inhibits HIF-1α protein levels through an Nrf2/PMF-1-mediated increase in spermidine/spermine acetyl transferase. Cancer Chemother Pharmacol. 2011;68(2):405–13.
Acknowledgments
The authors appreciate the joint financial support of this investigation by the Research Council of University of Tehran and the Iranian National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Sadeghirizi, A., Yazdanparast, R. & Aghazadeh, S. Combating trastuzumab resistance by targeting thioredoxin-1/PTEN interaction. Tumor Biol. 37, 6737–6747 (2016). https://doi.org/10.1007/s13277-015-4424-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4424-9