Abstract
Background
PE is a common pregnancy disease that can cause various pathologies in pregnant women. GEO microarray data revealed low expression of FGD5-AS1 in placenta tissues from patients with severe PE. As predicted by ENCORI, FGD5-AS1 sponges miR-16-5p, and IGF2, and promotes cell proliferation, differentiation, and metabolism.
Objectives
This study aimed to interrogate the interaction of FGD5-AS1/miR-16-5p/IGF2 axis and the mechanism of FGD5-AS1 in hypoxia-induced trophoblast cell injury.
Results
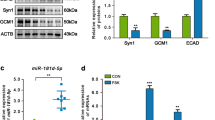
Our findings revealed that FGD5-AS1 and IGF2 were substantially decreased in PE patients than that in normal pregnant females, whereas miR-16-5p was increased in PE patients than that in normal pregnant females. In vitro studies showed that FGD5-AS1 were reduced in trophoblast cells after hypoxia treatment, accompanied by decreased viability and increased apoptosis, also reduced invasion, migration and angiogenesis. However, FGD5-AS1 overexpression substantially reversed these effects. Further mechanistic analysis showed that FGD5-AS1 could target miR-16-5p to regulate IGF2. FGD5-AS1 promoted the expression of IGF2 by sponging miR-16-5p, thereby increasing the viability of trophoblast cells after hypoxia treatment and promoting invasion and migration.
Conclusion
Overall, our findings demonstrated that FGD5-AS1 promoted growth and mobility of trophoblast cells by regulating miR-16-5p/IGF2 axis in PE.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abbas Y, Turco MY, Burton GJ, Moffett A (2020) Investigation of human trophoblast invasion in vitro. Hum Reprod Update 26:501–513
Burton GJ, Redman CW, Roberts JM, Moffett A (2019) Pre-eclampsia: pathophysiology and clinical implications. BMJ (clin Res Ed) 366:l2381
Chen L et al (2013) miR-16 inhibits cell proliferation by targeting IGF1R and the Raf1-MEK1/2-ERK1/2 pathway in osteosarcoma. FEBS Lett 587:1366–1372
Chow PH et al (2003) Embryos sired by males without accessory sex glands induce failure of uterine support: a study of VEGF, MMP and TGF expression in the golden hamster. Anat Embryol 206:203–213
Daimon E, Wada Y (2005) Role of neutrophils in matrix metalloproteinase activity in the preimplantation mouse uterus. Biol Reprod 73:163–171
Dubova EA et al (2014) Expression of insulin-like growth factors in the placenta in preeclampsia. B Exp Biol Med+ 157:103–107
Duley L (2009) The global impact of pre-eclampsia and eclampsia. Semin Perinatol 33:130–137
Gao Y et al (2019) The decreased lncRNA ZEB2-AS1 in pre-eclampsia controls the trophoblastic cell line HTR-8/SVneo’s invasive and migratory abilities via the miR-149/PGF axis. J Cell Biochem 120:17677–17686
Ge C et al (2020) LncRNA FGD5-AS1 promotes tumor growth by regulating MCL1 via sponging miR-153-3p in oral cancer. Aging 12:14355–14364
He J et al (2013) Methylation levels at IGF2 and GNAS DMRs in infants born to preeclamptic pregnancies. BMC Genom 14:472
Hu Y et al (2008) IFN-gamma-mediated extravillous trophoblast outgrowth inhibition in first trimester explant culture: a role for insulin-like growth factors. Mol Hum Reprod 14:281–289
Hu X et al (2019a) Expression profiling dataset of competing endogenous RNA in pre-eclampsia. Data Brief 27:104795–104795
Hu X et al (2019b) Expression profiling dataset of competing endogenous RNA in pre-eclampsia. Data Brief 27:104795
Hunt JS, Fishback JL (1989) Amniochorion: immunologic aspects–a review. Am J Reprod Immunol (new York, NY: 1989) 21:114–118
Irwin JC et al (1999) Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum Reprod 14:90–96
Liu J, Luo C, Zhang C (2020) Upregulated lncRNA UCA1 inhibits trophoblast cell invasion and proliferation by downregulating JAK2. J Cell Physiol 235:7410–7419
Liu G et al (2021) Activation of FGD5-AS1 promotes progression of cervical cancer through regulating BST2 to inhibit macrophage M1 polarization. J Immunol Res 2021:5857214
Ma J et al (2014) Novel death defying domain in met entraps the active site of caspase-3 and blocks apoptosis in hepatocytes. Hepatology 59:2010–2021
Mi C et al (2020) BHLHE40 plays a pathological role in pre-eclampsia through upregulating SNX16 by transcriptional inhibition of miR-196a-5p. Mol Hum Reprod 26:532–548
Mol BWJ et al (2016) Pre-eclampsia. Lancet (london, England) 387:999–1011
Nanaev A et al (1995) Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast. Cell Tissue Res 282:407–421
Salsoso R et al (2017) Adenosine and preeclampsia. Mol Aspects Med 55:126–139
Sandrim VC et al (2016) Plasma levels of increased miR-195-5p correlates with the sFLT-1 levels in preeclampsia. Hypertens Pregnancy 35:150–158
Thulluru HK, Michel OJ, Oudejans CB, van Dijk M (2015) ACVR2A promoter polymorphism rs1424954 in the Activin-A signaling pathway in trophoblasts. Placenta 36:345–349
Tranquilli AL et al (2013) The definition of severe and early-onset preeclampsia. Statements from the international society for the study of hypertension in pregnancy (ISSHP). Pregnancy Hypertens 3:44–47
Wang L, Zhang J (2021) Long intergenic ncRNA 00473 improves the invasion of trophoblastic cells via miR-16-5p. Pregnancy Hypertens 23:174–184
Wu L et al (2020) FGD5-AS1 facilitates glioblastoma progression by activation of Wnt/β-catenin signaling via regulating miR-129-5p/HNRNPK axis. Life Sci 256:117998
Yang Y et al (2020) LncRNA FGD5-AS1/miR-5590-3p axis facilitates the proliferation and metastasis of renal cell carcinoma through ERK/AKT signalling. Eur Rev Med Pharmacol Sci 24:8756–8766
Yuan Y et al (2020a) MicroRNA-16 is involved in the pathogenesis of pre-eclampsia via regulation of Notch2. J Cell Physiol 235:4530–4544
Yuan Y et al (2020b) Ligustrazine-induced microRNA-16-5p inhibition alleviates preeclampsia through IGF-2. Reprod (cambridge, England) 160:905–917
Yunusov D et al (2016) HIPSTR and thousands of lncRNAs are heterogeneously expressed in human embryos, primordial germ cells and stable cell lines. Sci Rep UK 6:32753
Zhang Qm et al (2020) Analysis of altered miRNA profiling in the colon of a mouse model with β-lactoglobulin allergy. Allergol Immunopath 48:666–674
Zhou Y et al (1993) Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest 91:950–960
Zhou F et al (2020) Long noncoding RNA SNHG12 promotes the proliferation, migration, and invasion of trophoblast cells by regulating the epithelial-mesenchymal transition and cell cycle. J Int Med Res 48(6):0300060520922339
Acknowledgements
Not applicable.
Funding
This work was supported by the Key Science and Technology Research Program of Jinhua City, Zhejiang Province (Grant No. 2021-3-120).
Author information
Authors and Affiliations
Contributions
LQ and YJ designed the study, supervised the data collection, JW analyzed the data and interpreted the data, XD, SZ, and ZS prepare the manuscript for publication and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A Limei Quan declares that he/she has no conflict of interest; Yan Jin declares that he/she has no conflict of interest; Jianfang Wang declares that he/she has no conflict of interest; Xiwang Dai declares that he/she has no conflict of interest; Shuli Zhu declares that he/she has no conflict of interest; Zengwei Sheng declares that he/she has no conflict of interest.
Ethical approval
Ethical approval was obtained from the Ethics Committee of Jinhua People’s Hospital (IRB-2020042-R).
Informed consent
Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quan, L., Jin, Y., Wang, J. et al. FGD5-AS1 promotes growth and mobility of trophoblast cells by regulating IGF2 via sponging miR-16-5p in pre-eclampsia. Mol. Cell. Toxicol. 20, 409–419 (2024). https://doi.org/10.1007/s13273-023-00357-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00357-y