Abstract
Alzheimer’s disease (AD) is one of the most commonly occurring forms of dementia in the elderly population worldwide. Despite the high prevalence of AD, diagnosis and treatment rates are poor. MicroRNAs (miRNAs) are non-coding RNA molecules consisting of about 19 to 25 nucleotides that serve as post-transcriptional regulators of gene expression. MiRNA dysfunction is closely linked to the formation of amyloid-β plaques and hyperphosphorylation of tau, both of which are the major hallmarks of AD. Thus, by knowing the miRNAs that are differentially expressed in AD patients as compared to normal healthy individuals, we can diagnose AD in patients during the early stages. In this review, we aim to address the role of miRNA in AD pathogenesis and consider how the differentially expressed miRNA or a sequence of miRNAs can serve as an early diagnostic biomarker for AD.
Graphical abstract
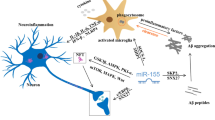
Role of MicroRNA associated with Alzheimer’s disease. Dysregulation of miRNA is associated with the formation and deposition of Amyloid-Beta Plaques as well as Neurofibrillary Tangles. Apart from this, it is also linked to impaired synaptic plasticity and increased expression of Beta-Amyloid Cleaving Enzyme 1(BACE1). All these eventually contribute to the progression of the disease.

Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.


Similar content being viewed by others
Abbreviations
- 18F-FDG:
-
18F labelled fluoro-2-deoxyglucose
- 2-AG:
-
2-Arachidonoylglycerol
- 3′-UTR:
-
3′ Untranslated regions
- ABP:
-
Amyloid-β plaques
- AD:
-
Alzheimer’s disease
- ADAM10:
-
A disintegrin and metalloproteinase domain-containing protein 10
- Add1:
-
Adducin1
- AGO:
-
Argonaute
- APP:
-
Amyloid precursor protein
- ATP:
-
Adenosine triphosphate
- Aβ:
-
Amyloid-β
- BACE1:
-
Beta-site amyloid precursor protein cleaving enzyme 1
- CNS:
-
Central nervous system
- CREB:
-
CAMP-response element binding protein
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- DGCR8:
-
DiGeorge syndrome critical region gene 8
- ERK1:
-
Extracellular signal-regulated kinase
- GSK-3β:
-
Glycogen synthase kinase-3 β
- IRS-1:
-
Insulin receptor substrate
- MAGL:
-
Monoacylglycerol lipase
- MAP1A:
-
Microtubule-associated protein 1A
- MAPK3:
-
Mitogen-activated protein kinase 3
- miRNA:
-
MicroRNA
- MMSE:
-
Mini-mental state examination
- MoCA:
-
Montreal cognitive assessment
- MRI:
-
Magnetic resonance imaging
- mRNA:
-
Messenger RNA
- mTOR:
-
Mammalian target of rapamycin
- NFT:
-
Neurofibrillary tangles
- NF-κB:
-
Nuclear factor kappa B
- PET:
-
Positron emission tomography
- PPARγ:
-
Peroxisome proliferator-activated receptor-γ
- pre-miRNA:
-
Precursor miRNA
- pri-miRNA:
-
Primary microRNA
- P-tau:
-
Phosphorylated tau
- PTEN:
-
Phosphatase and tensin homolog
- Ran:
-
Ras-related nuclear protein
- RISC:
-
RNA-induced silencing comlex
- Rock2:
-
Rho-associated coiled-coil containing protein kinase 2
- S6K1:
-
Substrate S6 kinase 1
- SAMP8:
-
Senescence accelerated mouse prone 8
- shRNA:
-
Short hairpin RNA
- SIRT1:
-
Sirtuin 1
- TG:
-
Transgenic
- TRBP:
-
Trans-activation response RNA-binding protein
- T-tau:
-
Total tau
- TTBK1:
-
Tau tubulin kinase 1
References
Abdelfattah AM, Park C, Choi MY. Update on non-canonical microRNAs. Biomol Concepts. 2014;5(4):275.
Abuelezz NZ, Nasr FE, AbdulKader MA, Bassiouny AR, Zaky A. MicroRNAs as potential orchestrators of Alzheimer’s disease-related pathologies: insights on current status and future possibilities. Front Aging Neurosci. 2021;13: 743573.
Ashrafian H, Zadeh EH, Khan RH. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int J Biol Macromol. 2021;167:382–94.
Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, Oates JA, Peck CC, Schooley RT, Spilker BA, Woodcock J, Zeger SL. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95.
Bjerke M, Engelborghs S. Cerebrospinal fluid biomarkers for early and differential Alzheimer’s disease diagnosis. J Alzheimer’s Dis. 2018;62:1199–209.
Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, de Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease–associated Aβ oligomers. J Clin Invest. 2012;122(4):1339–53.
Cao Y, Tan X, Lu Q, Huang K, Tang X, He Z. MiR-29c-3p may promote the progression of Alzheimer’s disease through BACE1. J Healthc Eng. 2021;2021:2031407.
Carrettiero DC, Hernandez I, Neveu P, Papagiannakopoulos T, Kosik KS. The cochaperone BAG2 sweeps paired helical filament- insoluble tau from the microtubule. J Neurosci. 2009;29(7):2151–61.
Chen X, Liang H, Zhang J, Zen K, Zhang CY. Horizontal transfer of microRNAs: molecular mechanisms and clinical applications. Prot Cell. 2012;3(1):28–37.
Cho E, Park JY. Emerging roles of 14-3-3γ in the brain disorder. BMB Rep. 2020;53(10):500.
Dakterzada F, David Benítez I, Targa A, Lladó A, Torres G, Romero L, de Gonzalo-Calvo D, Moncusí-Moix A, Tort-Merino A, Huerto R, Sánchez-de-la-Torre M, Barbé F, Piñol-Ripoll G. Reduced levels of miR-342-5p in plasma are associated with worse cognitive evolution in patients with mild Alzheimer’s disease. Front Aging Neurosci. 2021;13: 705989.
de Felice FG, Vieira MNN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Aβ oligomers. Proc Natl Acad Sci USA. 2009;106(6):1971–6.
Delacourte A, David JP, Sergeant N, Buée L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, di Menza C. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurolog. 1999;52(6):1158–1158.
Delay C, Calon F, Mathews P, Hébert SS. Alzheimer-specific variants in the 3’UTR of Amyloid precursor protein affect microRNA function. Mol Neurodegener. 2011;6(1):1–6.
Delay C, Hébert SS. MicroRNAs and Alzheimer’s disease mouse models: current insights and future research avenues. Int J Alzheimers Dis. 2011;2011: 894938.
El Fatimy R, Li S, Chen Z, Mushannen T, Gongala S, Wei Z, Balu DT, Rabinovsky R, Cantlon A, Elkhal A, Selkoe DJ. MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol. 2018;136(4):537–55.
FDA Permits Marketing for New Test to Improve Diagnosis of Alzheimer’s Disease | FDA [Internet]. [cited 2022 Jun 21]. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-new-test-improve-diagnosis-alzheimers-disease
Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105.
Geng L, Zhang T, Liu W, Chen Y. Inhibition of miR-128 abates Aβ-mediated cytotoxicity by targeting PPAR-γ via NF-κB inactivation in primary mouse cortical neurons and Neuro2a cells. Yonsei Med J. 2018;59(9):1096.
Ghafouri-Fard S, Shoorei H, Bahroudi Z, Abak A, Majidpoor J, Taheri M. An update on the role of miR-124 in the pathogenesis of human disorders. Biomed Pharmacother. 2021;135:1.
Guo R, Fan G, Zhang J, Wu C, Du Y, Ye H, Li Z, Wang L, Zhang Z, Zhang L, Zhao Y, Lu Z. A 9-microRNA signature in serum serves as a noninvasive biomarker in early diagnosis of Alzheimer’s disease. J Alzheimers Dis. 2017;60(4):1365–77.
Hébert SS, Papadopoulou AS, Smith P, Galas MC, Planel E, Silahtaroglu AN, Sergeant N, Buée L, de Strooper B. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum Mol Genet. 2010;19(20):3959–69.
Hernández F, Cuadros R, Avila J. Zeta 14-3-3 protein favours the formation of human tau fibrillar polymers. Neurosci Lett. 2004;357(2):143–6.
Higaki S, Muramatsu M, Matsuda A, Matsumoto K, Satoh J, Michikawa M, Niida S. Defensive effect of microRNA-200b/c against amyloid-beta peptide-induced toxicity in Alzheimer’s disease models. PLoS One 2018;13(5):e0196929.
Hippius H, Neundörfer G. The discovery of Alzheimer’s disease. Dialogues Clin Neurosci. 2003;5(1):101.
Hung ASM, Liang Y, Chow TCH, Tang HC, Wu SLY, Wai MSM, Yew DT. Mutated tau, amyloid and neuroinflammation in Alzheimer disease—a brief review. Prog Histochem Cytochem. 2016;51(1):1–8.
Inestrosa NC, Tapia-Rojas C, Griffith TN, Carvajal FJ, Benito MJ, Rivera-Dictter A, Alvarez AR, Serrano FG, Hancke JL, Burgos PV, Parodi J, Varela-Nallar L. Tetrahydrohyperforin prevents cognitive deficit, Aβ deposition, tau phosphorylation and synaptotoxicity in the APPswe/PSEN1ΔE9 model of Alzheimer’s disease: a possible effect on APP processing. Transl Psychiatry. 2011;1(7):e20.
Jiang H, Liu J, Guo S, Zeng L, Cai Z, Zhang J, Wang L, Li Z, Liu R. miR-23b-3p rescues cognition in Alzheimer’s disease by reducing tau phosphorylation and apoptosis via GSK-3β signaling pathways. Mol Ther Nucl Acids. 2022;28:539–57.
Joo Y, Schumacher B, Landrieu I, Bartel M, Smet-Nocca C, Jang A, Choi HS, Jeon NL, Chang KA, Kim HS, Ottmann C, Suh YH. Involvement of 14-3-3 in tubulin instability and impaired axon development is mediated by Tau. FASEB J. 2015;29(10):4133–44.
Khoury R, Ghossoub E. Diagnostic biomarkers of Alzheimer’s disease: A state-of-the-art review. Biomark Neuropsychiatry. 2019;1: 100005.
Kumar S, Reddy PH. Are circulating microRNAs peripheral biomarkers for Alzheimer’s disease? Biochim Biophys Acta. 2016;1862(9):1617–27.
Kumar S, Reddy PH. The role of synaptic microRNAs in Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12).
Kurt MA, Davies DC, Kidd M, Duff K, Howlett DR. Hyperphosphorylated tau and paired helical filament-like structures in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Neurobiol Dis. 2003;14(1):89–97.
Lee RC, Feinbaum RL, Ambrost V. The C. elegans Heterochronic Gene lin-4 Encodes Small RNAs with Antisense Complementarity to &II-14. Cell. 1993;75:843–54.
Liu Y, Zhang Y, Liu P, Bai H, Li X, Xiao J, Yuan Q, Geng S, Yin H, Zhang H, Wang Z, Li J, Wang S, Wang Y. MicroRNA-128 knockout inhibits the development of Alzheimer’s disease by targeting PPARγ in mouse models. Eur J Pharmacol. 2019;843:134–44.
Long JM, Maloney B, Rogers JT, Lahiri DK. Novel upregulation of amyloid-β precursor protein (APP) by microRNA-346 via targeting of APP mRNA 5′-untranslated region: Implications in Alzheimer’s disease. Mol Psychiatry. 2018;24(3):345–63.
Long JM, Ray B, Lahiri DK. MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J Biol Chem. 2014;289(8):5184–98.
Lu TX, Rothenberg ME. Fundamentals of allergy and immunology MicroRNA. J Allergy Clin Immunol. 2018;141:1202–7.
Ma T, Sun X, Sun S, Guo R, Ma X. The study of peripheral blood miR-29a/101 in the diagnosis of Alzheimer’s disease. Chin J Behav Med Brain Sci. 2016;11:1010–4.
Miyoshi K, Miyoshi T, Siomi H. Many ways to generate microRNA-like small RNAs: non-canonical pathways for microRNA production. Mol Genet Genomics. 2010;284(2):95–103.
Monserrate AE, Ryman DC, Ma S, Xiong C, Noble JM, Ringman JM, Morris JC, Danek A, Müller-Sarnowski F, Clifford DB, McDade EM, Brooks WS, Darby DG, Masters CL, Weston PSJ, Farlow MR, Graff-Radford NR, Salloway SP, Fagan AM, Oliver A, Bateman RJ, Dominantly Inherited Alzheimer Network. Factors associated with the onset and persistence of post-lumbar puncture headache. JAMA Neurol. 2015;72(3):325–32.
Nagaraj S, Zoltowska KM, Laskowska-Kaszub K, Wojda U. microRNA diagnostic panel for Alzheimer’s disease and epigenetic trade-off between neurodegeneration and cancer. Ageing Res Rev. 2019;49:125–43.
O’Bryant SE, Gupta V, Henriksen K, Edwards M, Jeromin A, Lista S, Bazenet C, Soares H, Lovestone S, Hampel H, Montine T, Blennow K, Foroud T, Carrillo M, Graff-Radford N, Laske C, Breteler M, Shaw L, Trojanowski JQ, Schupf N, Rissman RA, Fagan AM, Oberoi P, Umek R, Weiner MW, Grammas P, Posner H, Martins R. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer’s disease research. Alzheimers Dement. 2015;11(5):549–60.
Pair FS, Yacoubian TA. 14-3-3 Proteins: novel pharmacological targets in neurodegenerative diseases. Trends Pharmacol Sci. 2021;42(4):226.
Park H, Lee YB, Chang KA. miR-200c suppression increases tau hyperphosphorylation by targeting 14-3-3γ in early stage of 5xFAD mouse model of Alzheimer’s disease. Int J Biol Sci. 2022;18(5):2220.
Pereira JB, Janelidze S, Ossenkoppele R, Kvartsberg H, Brinkmalm A, Mattsson-Carlgren N, Stomrud E, Smith R, Zetterberg H, Blennow K, Hansson O. Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer’s disease. Brain. 2021;144(1):310–24.
Peskind E, Nordberg A, Darreh-Shori T, Soininen H. Safety of lumbar puncture procedures in patients with Alzheimer’s disease. Curr Alzheimer Res. 2009;6(3):290.
Qureshi HY, Han D, MacDonald R, Paudel HK. Overexpression of 14-3-3ζ promotes tau phosphorylation at Ser262 and accelerates proteosomal degradation of synaptophysin in rat primary hippocampal neurons. PLoS ONE. 2013;8(12): e84615.
Rapado-González Ó, Álvarez-Castro A, López-López R, Iglesias-Canle J, Suárez-Cunqueiro MM, Muinelo-Romay L. Circulating microRNAs as Promising Biomarkers in Colorectal Cancer. Cancers (Basel). 2019;11(7):1.
Reddy PH, Tonk S, Kumar S, Vijayan M, Kandimalla R, Kuruva CS, Reddy AP. A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer’s disease. Biochem Biophys Res Commun. 2017;483(4):1156–65.
Rodriguez-Ortiz CJ, Baglietto-Vargas D, Martinez-Coria H, Laferla FM, Kitazawa M. Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J Alzheimer’s Dis. 2014;42(4):1229–38.
Rodriguez-Ortiz CJ, Prieto GA, Martini AC, Forner S, Trujillo-Estrada L, LaFerla FM, Baglietto-Vargas D, Cotman CW, Kitazawa M. miR-181a negatively modulates synaptic plasticity in hippocampal cultures and its inhibition rescues memory deficits in a mouse model of Alzheimer’s disease. Aging Cell. 2020;19(3):1.
Sadik G, Tanaka T, Kato K, Yamamori H, Nessa BN, Morihara T, Takeda M. Phosphorylation of tau at Ser214 mediates its interaction with 14-3-3 protein: implications for the mechanism of tau aggregation. J Neurochem. 2009;108(1):33–43.
Schonrock N, Götz J. Decoding the non-coding RNAs in Alzheimer’s disease. Cell Mol Life Sci. 2012;69(21):3543–59.
Smith P, al Hashimi A, Girard J, Delay C, Hébert SS,. In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs. J Neurochem. 2011;116(2):240–7.
Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82(4):756.
Srivastava S, Ahmad R, Khare SK. Alzheimer’s disease and its treatment by different approaches: A review. Eur J Med Chem. 2021;216: 113320.
Sun T, Zhao K, Liu M, Cai Z, Zeng L, Zhang J, Li Z, Liu R. miR-30a-5p induces Aβ production via inhibiting the nonamyloidogenic pathway in Alzheimer’s disease. Pharmacol Res. 2022;178: 106153.
Swarbrick S, Wragg N, Ghosh S, Stolzing A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol Neurobiol. 2019;56(9):6156–67.
Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–35.
Tonda-Turo C, Origlia N, Mattu C, Accorroni A, Chiono V. Current limitations in the treatment of Parkinson’s and Alzheimer’s diseases: state-of-the-art and future perspective of polymeric carriers. Curr Med Chem. 2018;25(41):5755–71.
Torromino G, Maggi A, de Leonibus E. Estrogen-dependent hippocampal wiring as a risk factor for age-related dementia in women. Prog Neurobiol. 2021;197: 101895.
van den Berg MMJ, Krauskopf J, Ramaekers JG, Kleinjans JCS, Prickaerts J, Briedé JJ. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog Neurobiol. 2020;185: 101732.
Vilming ST, Kloster R, Sandvik L. The importance of sex, age, needle size, height and body mass index in post-lumbar puncture headache. Cephalalgia. 2001;21(7):738–43.
Wang L, Liu J, Wang Q, Jiang H, Zeng L, Li Z, Liu R. MicroRNA-200a-3p mediates neuroprotection in Alzheimer-related deficits and attenuates amyloid-beta overproduction and tau hyperphosphorylation via coregulating BACE1 and PRKACB. Front Pharmacol. 2019;1:806.
Wang T, Xiao S, Liu Y, Lin Z, Su N, Li X, Li G, Zhang M, Fang Y. The efficacy of plasma biomarkers in early diagnosis of Alzheimer’s disease. Int J Geriatr Psychiatry. 2014;29(7):713–9.
Wu HZY, Thalamuthu A, Cheng L, Fowler C, Masters CL, Sachdev P, Mather KA. Differential blood miRNA expression in brain amyloid imaging-defined Alzheimer’s disease and controls. Alzheimers Res Ther. 2020;12(1):59.
Wu Y, Xu J, Xu J, Cheng J, Jiao D, Zhou C, Dai Y, Chen Q. Lower serum levels of miR-29c-3p and miR-19b-3p as biomarkers for Alzheimer’s disease. Tohoku J Exp Med. 2017;242(2):129–36.
Yang G, Song Y, Zhou X, Deng Y, Liu T, Weng G, Yu D, Pan S. MicroRNA-29c targets β-site amyloid precursor protein-cleaving enzyme 1 and has a neuroprotective role in vitro and in vivo. Mol Med Rep. 2015;12(2):3081–8.
Zeng L, Jiang H, Ashraf GM, Liu J, Wang L, Zhao K, Liu M, Li Z, Liu R. Implications of miR-148a-3p/p35/PTEN signaling in tau hyperphosphorylation and autoregulatory feedforward of Akt/CREB in Alzheimer’s disease. Mol Ther Nucleic Acids. 2022;27:256–75.
Zhang J, Hu M, Teng Z, Tang YP, Chen C. Synaptic and Cognitive Improvements by Inhibition of 2-AG Metabolism Are through Upregulation of MicroRNA-188-3p in a Mouse Model of Alzheimer’s Disease. J Neurosci. 2014;34(45):14919.
Zhang M, Han W, Xu Y, Li D, Xue Q. Serum miR-128 Serves as a Potential Diagnostic Biomarker for Alzheimer’s Disease. Neuropsychiatr Dis Treat. 2021;17:269.
Zhang N, Li WW, Lv CM, Gao YW, Liu XL, Zhao L. MiR-16–5p and miR-19b-3p prevent amyloid β-induced injury by targeting BACE1 in SH-SY5Y cells. Neuroreport. 2020;205–12.
Zhan-Qiang H, Hai-Hua Q, Chi Z, Miao W, Cui Z, Zi-Yin L, Jing H, Yi-Wei W. miR-146a aggravates cognitive impairment and Alzheimer disease-like pathology by triggering oxidative stress through MAPK signaling. Neurologia (Engl Ed). 2021 11:S0213–4853(21)00022–0.
Zheng K, Hu F, Zhou Y, Zhang J, Zheng J, Lai C, Xiong W, Cui K, Hu YZ, Han ZT, Zhang HH, Chen JG, Man HY, Liu D, Lu Y, Zhu LQ. miR-135a-5p mediates memory and synaptic impairments via the Rock2/Adducin1 signaling pathway in a mouse model of Alzheimer’s disease. Nat Commun. 2021;12(1):1.
Acknowledgements
Logistic support from SVKM’s Dr. Bhanuben Nanavati College of Pharmacy, Mumbai, and Institute of Chemical Technology, Mumbai, India, is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
RCS and AG have conceptualized the idea and prepared the manuscript. AG supervised the writing process for the review article and both authors have finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Consent for publication
We hereby give consent for the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Editor: Ronita Nag Chaudhuri; Reviewer: Somnath Paul.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sequeira, R.C., Godad, A. An update on microRNA as a potential blood-based biomarker for Alzheimer’s disease. Nucleus (2023). https://doi.org/10.1007/s13237-023-00427-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13237-023-00427-5