Abstract
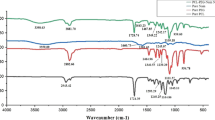
As synthetic medications have various limitations and side effects in wound treatment, alternatively active wound dressing material could be designed by incorporating natural extracts. Electrospun fibers infused with plant extract can act as an active wound dressing material to speed up the process of wound healing. In this connection, the wound-healing efficacy of an Indian traditional drug Morinda tinctoria was reported earlier, but not yet explored as a natural active ingredient in electrospun fibers. The present work focused on preparing the extracts of M. tinctoria leaf and analyzing their yield, total phenolic concentration (TPC) and GC–MS phytochemical profile. The safety of methanol extract was studied in PBMC cells through MTT and live/dead assays and also TNF-α & IL-6 levels were quantified. Electrospinning of M. tinctoria-infused PHBV fiber mat was developed and characterized through SEM, FT-IR and TG–DTA. Results indicated that the methanol extract has a greater yield (2.47%) and TPC (4260.72 mg/L) when compared to other solvent extracts due to the presence of high polar molecules in M. tinctoria. GC–MS study revealed the phytochemicals such as coumarin and methylanthraquinone in the methanolic extract. MTT assay and live/dead staining revealed that the M. tinctoria extract was safe with 99% cell viability with intact cell wall. M. tinctoria induced the levels of TNF-α (328.75 pg/mL) and IL-6 (1357.14 pg/mL), which could improve the migration and proliferation of fibroblasts during wound healing. Development of M. tinctoria-infused fiber mat was optimized with 12% polymer concentration, 18 kV voltage, 0.01 mL/min flow rate and 14 cm tip to collector distance. SEM analysis of M. tinctoria-infused fiber mat revealed bead-less smooth fibers of 1.19 ± 0.15 µm diameter and 0.024 ± 0.003 µm pore size. FT-IR spectra revealed functional groups such as phenols, amines and alkanes in extract-incorporated fibers. A drop in temperature (457 °C) was noticed in M. tinctoria-infused fiber mat when compared to the control (595 °C), which indicates the degradation of organic matters in the fiber mat. M. tinctoria-infused fiber mat could be applied as wound dressing material after conducting suitable animal experiments.
Graphical abstract








Similar content being viewed by others
References
Barbieri J.S., Wanat K., Seykora J, Skin: Basic structure and function. In: Pathobiology of human disease. Academic Press. ISBN 9780123864574. 2014
P. Bainbridge, Wound healing and the role of fibroblasts. J. Wound Care 22(8), 407–412 (2013). https://doi.org/10.12968/jowc.2013.22.8.407
P. Kujath, A. Michelsen, Wounds—from physiology to wound dressing. Dtsch. Arztebl. Int. 105(13), 239–248 (2008). https://doi.org/10.3238/arztebl.2008.0239
R.S. Kirsner, W.H. Eaglstein, The wound healing process. Dermatol. Clin. 11(4), 629–640 (1993). https://doi.org/10.1016/S0733-8635(18)30216-X
A. Sharma, S. Khanna, G. Kaur, Medicinal plants and their components for wound healing applications. Fut J Pharm Sci 7, 53 (2021). https://doi.org/10.1186/s43094-021-00202-w
I. Onyekwelu, R. Yakkanti, L. Protzer, C.M. Pinkston, C. Tucker, D. Seligson, Surgical wound classification and surgical site infections in the orthopaedic patient. JAAOS Global Res Rev 1(3), 22 (2017). https://doi.org/10.5435/JAAOSGlobal-D-17-00022
S. Guo, L.A. DiPietro, Factors affecting wound healing. J. Dent. Res. 89(3), 219–229 (2010)
P.A. Than, C.R. Davis, C. Geoffrey Gurtner, Gurtner Chapter 4 Clinical management of wound healing and hypertrophic scarring (Skin Tissue Engineering and Regenerative Medicine. Academic Press., Cambridge USA, 2016), pp.61–81
G.C. Gurtner, S. Werner, Y. Barrandon, M.T. Longaker, Wound repair and regeneration. Nature 453(7193), 314–321 (2008). https://doi.org/10.1038/nature07039
S. Enoch, D.J. Leaper, Basic science of wound healing. Surg. Infect. (Larchmt.) 26(2), 31–37 (2008). https://doi.org/10.1016/j.mpsur.2007.11.005
M. Yokota, N. Häffner, M. Kassier, Staphylococcus aureus impairs dermal fibroblast functions with deleterious effects on wound healing. FASEB J. 35(7), 21695 (2021). https://doi.org/10.1096/fj.201902836R
A. Hecker, M. Schellnegger, E. Hofmann, The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 19(1), 9–28 (2022). https://doi.org/10.1111/iwj.13601
R.C. Canada, Á. Bernabé-García, S. Liarte, M. Rodríguez-Valiente, F.J. Nicolás, Chronic wound healing by amniotic membrane: TGF-β and EGF signaling modulation in re-epithelialization. Front Bioeng Biotechnol. 9, 689328 (2021)
K. Muniandy, S. Gothai, W.S. Tan, In vitro wound healing potential of stem extract of Alternanthera sessilis. Evid Based Complement Altern Med. 2018, 3142073 (2018). https://doi.org/10.1155/2018/3142073
H. Nakao, M. Yamazaki, R. Tsuboi, H. Ogawa, Mixture of sugar and povidone-iodine stimulates wound healing by activating keratinocytes and fibroblast functions. Archie Dermatol Res 298(4), 175–182 (2006). https://doi.org/10.1007/s00403-006-0683-z
M.S. Gurel, S. Naycı, A.V. Turgut, E.R. Bozkurt, Comparison of the effects of topical fusidic acid and rifamycin on wound healing in rats. Int. Wound J. 12(1), 106–110 (2015). https://doi.org/10.1111/iwj.12060
M.G. Cordes, Povidone xPharm: the comprehensive pharmacology reference (Elsevier, Amsterdam Netherlands, 2007), pp.1–3
H. Schofer, L. Simonsen, Fusidic acid in dermatology: an updated review. Eur. J. Dermatol. 20(1), 6–15 (2010). https://doi.org/10.1684/ejd.2010.0833
A. Shedoeva, D. Leavesley, Z. Upton, C. Fan, Wound healing and the use of medicinal plants. Evid Based Complement Altern Med. 2019, 1–30 (2019). https://doi.org/10.1155/2019/2684108
S. Razia, H. Park, E. Shin, Synergistic effect of Aloe vera flower and Aloe gel on cutaneous wound healing targeting MFAP4 and its associated signaling pathway: In vitro study. J Ethnopharmacol. 290, 115096 (2022). https://doi.org/10.1016/j.jep.2022.115096
X. Dai, J. Liu, H. Zheng, J. Wichmann, U. Hopfner, S. Sudhop, C. Prein, Y. Shen, H.G. Machens, A. Schilling, Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater 9, 368 (2017). https://doi.org/10.1038/am.2017.31
U.I. Ivens, B. Steinkjer, J. Serup, V. Tetens, Ointment is evenly spread on the skin, in contrast to creams and solutions. Br. J. Dermatol. 145(2), 264–267 (2001). https://doi.org/10.1046/j.1365-2133.2001.04344.x
R. Augustine, N. Kalarikkal, S. Thomas, Advancement of wound care from grafts to bioengineered smart skin substitutes. Prog. Biomater. 3(2–4), 103–113 (2014). https://doi.org/10.1007/s40204-014-0030-y
R.M. Marziyeh, R. Shahram, S.H. Bahrami, M.T. Joghataei, F. Moayer, Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth / poly(ε-caprolactone) electrospun nanofibers. Mater Sci Eng-C Mater Biolog Appl 69, 1183–1191 (2016). https://doi.org/10.1016/j.msec.2016.08.032
H.S. Bae, A. Haider, K. Selim, D.Y. Kang, E.J. Kim, I.K. Kang, Fabrication of highly porous PMMA electrospun fibers and their application in the removal of phenol and iodine. J. Polym. Res. 20, 158 (2013). https://doi.org/10.1007/s10965-013-0158-9
P.A. Gunatillake, R. Adhikari, Biodegradable synthetic polymers for tissue engineering. Eur. Cell. Mater. 5, 1–16 (2003). https://doi.org/10.22203/ecm.v005a01
A.L. Rivera-Briso, Á. Serrano-Aroca, Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): enhancement strategies for advanced applications. Polymers (Basel) 10(7), 732 (2018). https://doi.org/10.3390/polym10070732
M. Koller, A. Salerno, M. Dias, A. Reiterer, G. Braunegg, Modern biotechnological polymer synthesis: a review. Food Technol Biotechnol 48, 255–269 (2010)
H. Adeli, M.T. Khorasani, M. Parvazinia, Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Intern J Biol Macromole 122, 238–254 (2019). https://doi.org/10.1016/j.ijbiomac.2018.10.115
S.K. Karuppannan, J. Bushion, R. Ramalingam, S. Swaminathan, K.D. Arunachalam, A.A. Kadam, R. Rajagopal, R. Sathya, S. Chinnappan, Fabrication, characterization and in vitro evaluation of Melia dubia extract infused nanofibers for wound dressing. J King Saud Univ Sci. 34(4), 101931 (2022). https://doi.org/10.1016/j.jksus.2022.101931
Kirtikar K.R.B.B., Basu B.D., Indian medicinal plants. Indian Medicinal Plants. 2, 2nd edn. (K.A. Longman, Dehradun, 1935) 1294–1295.
K. Deepti, P. Umadevi, G. Vijayalakshmi, Antimicrobial activity and phytochemical analysis of Morinda tinctoria Roxb leaf extracts. Asian Pac. J. Trop. Biomed. 2(3), 1440–1442 (2012)
M. Subramanian, S. Balakrishnan, S.K. Chinnaiyan, V.K. Sekar, A.N. Chandu, Hepatoprotective effect of leaves of Morinda tinctoria Roxb. Against paracetamol induced liver damage in rats. Drug Invent Today. 5(3), 223–228 (2013)
J.R.S. Rex, G. Gnanavel, M. Muthukumar Nadar, R. Sankaranarayanan, Wound healing activity of Morinda tinctoria Roxb aqueous leaf extract. Biotech 8(8), 343 (2018)
N. Mathivanan, G. Surendiran, K. Srinivasan, K. Malarvizhi, Morinda pubescens JE Smith (Morinda tinctoria Roxb) fruit extract accelerates wound healing in rats. J. Med. Food 9(4), 591–593 (2006)
K. Nivedha, S. Sivasakthi, A. Prakash, N. Devipriya, V. Vadivel, In vitro studies on antioxidant and cyto-protective activities of polyphenol-rich fraction isolated from Mangifera indica leaf. S. Afr. J. Bot. 130, 396–406 (2020)
A. Abirami, S. Sinsinwar, P. Rajalakshmi, P. Brindha, Y.B.R.D. Rajesh, V. Vadivel, Antioxidant and cytoprotective properties of loganic acid isolated from seeds of Strychnos potatorum L. against heavy metal induced toxicity in PBMC model. Drug Chem Toxicol. 45(1), 239–249 (2022). https://doi.org/10.1080/01480545.2019.1681445
S. Surendhiran, M.M. Arjun, B. Steven, P. Rajalakshmi, V. Vadivel, Protective effect of eugenol from Mesua ferrea on the oxidative damages caused by 5-fluorouracil in PBMC cells. Indian J. Nat. Prod. Resour. 13(1), 78–85 (2022)
S. Sinsinwar, V. Vadivel, Development and characterization of catechin-in-cyclodextrin-in-phospholipid liposome to eradicate MRSA-mediated surgical site infection: investigation of their anti-infective efficacy through in vitro and in vivo studies. Int. J. Pharm. 609, 121130 (2021)
D. Sundaramurthi, K.S. Vasanthan, P. Kuppan, U.M. Krishnan, S. Sethuraman, Electrospun nanostructured chitosan-poly(vinyl alcohol) scaffolds: a biomimetic extracellular matrix as dermal substitute. Biomed. Mater. 7(4), 045005 (2012)
S. Shanmugavel, T. Ram, B. Lakshmi, V. Giridev, Herbal drug incorporated antibacterial nanofibrous mat fabricated by electrospinning: an excellent matrix for wound dressings. J. Appl. Polym. Sci. 121, 2893–2899 (2011)
S. Shewale, V.K. Rathod, Extraction of total phenolic content from Azadirachta indica or (neem) leaves: Kinetics study. Prep. Biochem. Biotechnol. 48(4), 312–320 (2018)
T. Sivakumar, B.S. Sivamaruthi, K.L. Priya, P. Kesika, C. Chaiyasut, Evaluation of bioactivities of Morinda tinctoria leaves extract for pharmacological applications. Asian J Pharmaceut Clin Res. 11(2), 100–105 (2018)
D. Kolli, K.R. Amperayani, U. Parimi, Total phenolic content and antioxidant activity of Morinda tinctoria Leaves. Indian J. Pharm. Sci. 77(2), 226–230 (2015). https://doi.org/10.4103/0250-474x.156616
I. Pathak, M. Niraula, Assessment of total phenolic, flavonoid content and antioxidant activity of Ocimum sanctum Linn. J. Nepal Chem. Soc. 40, 30–35 (2019)
M.J. Matos, S. Vazquez-Rodriguez, L. Santana, E. Uriarte, C. Fuentes-Edfuf, Y. Santos, Synthesis and structure-activity relationships of novel amino/nitro substituted 3-arylcoumarins as antibacterial agents. Molecules 18(2), 1394–1404 (2013)
Y. Bansal, P. Sethi, G. Bansal, Coumarin: a potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 22(7), 3049–3060 (2013)
G.B. Bubols, D. Vianna, A. Medina-Remon, G. Poser, R.M. Lamuela-Raventos, V.L. Eifler-Lima, S.C. Garcia, The antioxidant activity of coumarins and flavonoids. Mini. Rev. Med. Chem. 13(3), 318–334 (2013)
H. Maryam, M. Afshar, M. Hassanzadeh-Taheri, M. Zardast, Efficacy of topical application of coumarin on incisional wound healing in BALB/c mice. Iran J Dermatol 23(2), 56–63 (2020)
R. Sarah, B. Tabassum, N. Idrees, M.K. Hussain, Bio-active compounds isolated from neem tree and their applications. Nat Bio-active Compd (2019). https://doi.org/10.1007/978-981-13-7154-7_17
S. Irshad, A. Muazzam, Z. Shahid, D.M. Baxter, Curcuma longa (Turmeric): an auspicious spice for antibacterial, phytochemical and antioxidant activities. Pak. J. Pharm. Sci. 31, 2689–2696 (2018)
Z.M. Song, J.L. Zhang, K. Zhou, L.M. Yue, Y. Zhang, C.Y. Wang, K.L. Wang, Y. Xu, Anthraquinones as potential antibiofilm agents against methicillin-resistant Staphylococcus aureus. Front Microbiol. 12, 709826 (2021). https://doi.org/10.3389/fmicb.2021.709826
J.M. Badr, Antioxidant and antimicrobial constituents of Crucianella maritima L. Nat. Prod. Sci. 14(4), 227–232 (2008)
M. Nyeem, M.D. Mannan, Rubia cordifolia - phytochemical and pharmacological evaluation of indigenous medicinal plants: a review. Intern J Physiol Nut Phys Edu 3(1), 766–771 (2018)
F. Attari, M. Zahmatkesh, H. Aligholi, S.E. Mehr, M. Sharifzadeh, A. Gorji, T. Mokhtari, M. Khaksarian, G. Hassanzadeh, Curcumin as a double-edged sword for stem cells: dose, time and cell type-specific responses to curcumin. Daru J Faculty Pharm Tehran Univ Med Sci. 23(1), 33 (2015)
B.A. Mast, G.S. Schultz, Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Rep Regen 4(4), 411–420 (1996)
B.Z. Johnson, A.W. Stevenson, C.M. Prêle, M.W. Fear, F.M. Wood, The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 8(5), 101 (2020)
D. Dwivedi, M. Dwivedi, S. Malviya, V. Singh, Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J. Tradit. Complement. Med. 7(1), 79–85 (2017). https://doi.org/10.1016/j.jtcme.2015.12.002
T. Sun, D. Norton, A.J. Ryan, S. MacNeil, J.W. Haycock, Investigation of fibroblast and keratinocyte cell-scaffold interactions using a novel 3D cell culture system. J. Mater. Sci. Mater. Med. 18, 321–328 (2007)
V.S. Reddy, Y. Tian, C. Zhang, Z. Ye, K. Roy, A. Chinnappan, S. Ramakrishna, W. Liu, R. Ghosh, A review on electrospun nanofibers based advanced applications: from health care to energy devices. Polymers 13(21), 3746 (2021)
R. Rajalakshmi, M. Andiappan, M. Muthalagu, Preparation and characterization of Terminalia bellerica loaded PCL nanofibrous mats for biomedical applications. Materials Today Proc 45, 7247–7252 (2021). https://doi.org/10.1016/j.matpr.2021.03.021
O. Suwantong, U. Ruktanonchai, P. Supaphol, Electrospun cellulose acetate fiber mats containing asiaticoside or Centella asiatica crude extract and the release characteristics of asiaticoside. Polymer 49(19), 4239–4247 (2008). https://doi.org/10.1016/j.polymer.2008.07.020
S. Sekaran, S. Vimalraj, G. Lakshmanan, A. Jindal, D. Sundaramurthi, J. Bhattacharya, Chitosan-based biocomposite scaffolds and hydrogels for bone tissue regeneration. Spring Ser Biomater Sci Eng. (2019). https://doi.org/10.1007/978-981-13-8855-2_18
G. Jin, M.P. Prabhakaran, D. Kai, S.K. Annamalai, K.D. Arunachalam, S. Ramakrishna, Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials 34(3), 724–734 (2013)
R. Naphade, J. Jog, Electrospinning of PHBV/ZnO membranes: structure and properties. Fib Poly 13(6), 692–697 (2012)
K. Salima, N. Le Moigne, M. Kaci, Morphological characterization and thermal properties of compatibilized poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/poly(butylene succinate) (PBS)/halloysite ternary nanocomposites. Eur. Polymer J. 75, 142–162 (2016). https://doi.org/10.1016/j.eurpolymj.2015.12.009
R.A. ShroogShdied, M.A. Malik, S.A. Al-thabaiti, Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo red dye degradation. J. Market. Res. 9(5), 10031–10044 (2020). https://doi.org/10.1016/j.jmrt.2020.06.074
M.K.A. Araruna, K.K.A. Santos, J.G.M. Costa, H.D.M. Coutinho, A.A. Boligon, S.T. Stefanello, M.L. Athayde, R.A. Saraiva, J.B.T. Rocha, M.R. Kerntopf, I.R.A. Menezes, Phenolic composition and in vitro activity of the Brazilian fruit tree Caryocar coriaceum Wittm. Europ J Integrat Med 5(2), 178–183 (2013). https://doi.org/10.1016/j.eujim.2012.11.007
R. Mazzei, M. Leonti, S. Spadafora, A. Patitucci, G. Tagarelli, A review of the antimicrobial potential of herbal drugs used in popular Italian medicine (1850s–1950s) to treat bacterial skin diseases. J Ethnopharmacol. 250, 112443 (2020). https://doi.org/10.1016/j.jep.2019.112443
Acknowledgements
Authors are thankful to the Management of SASTRA Deemed University, Thanjavur, Tamilnadu for their encouragement and support.
Funding
This research work was not supported by any Governmental / Non-Governmental / Public/Private funding agencies.
Author information
Authors and Affiliations
Contributions
JSN, SV, RS and MPS: performed the experiments, collected data, interpreted the results & wrote the draft of the article; DS and VV: developed the concept, designed the work, supervised & made a critical revision of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests and all authors agreed and approved the content of the manuscript.
Ethical approval
The authors obtained permission from the Institutional Bio-safety Committee (IBSC Ref. No. SASTRA/IBSC/8/2021 Dated: 15/12/2021) for working with a human pathogen (MRSA). There are no animal / human studies reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nissi, J.S., Vyaishnavi, S., Sivaranjanee, R. et al. Development and characterization of Morinda tinctoria incorporated electrospun PHBV fiber mat for wound healing application. Macromol. Res. 31, 393–405 (2023). https://doi.org/10.1007/s13233-023-00149-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-023-00149-2