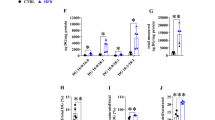
Abstract
O-GlcNAcylation, a nutritionally driven, post-translational modification of proteins, is gaining importance because of its health implications. Changes in O-GlcNAcylation are observed in various disease conditions. Changes in O-GlcNAcylation by diet that causes hypercholesterolemia are not critically looked into in the liver. To address it, both in vitro and in vivo approaches were employed. Hypercholesterolemia was induced individually by feeding cholesterol (H)/high-fat (HF) diet. Global O-GlcNAcylation levels and modulation of AMPK activation in both preventive and curative approaches were looked into. Diet-induced hypercholesterolemia resulted in decreased O-GlcNAcylation of liver proteins which was associated with decreased O-linked N-acetylglucosaminyltransferase (OGT) and Glutamine fructose-6-phosphate amidotransferase-1 (GFAT1). Activation of AMPK by metformin in preventive mode restored the O-GlcNAcylation levels; however, metformin treatment of HepG2 cells in curative mode restored O-GlcNAcylation levels in HF but failed to in H condition (at 24 h). Further, maternal faulty diet resulted in decreased O-GlcNAcylation in pup liver despite feeding normal diet till adulthood. A faulty diet modulates global O-GlcNAcylation of liver proteins which is accompanied by decreased AMPK activation which could exacerbate metabolic syndromes through fat accumulation in the liver.
Graphical abstract










Similar content being viewed by others
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- ACC:
-
Acetyl-CoA carboxylase
- C:
-
Control
- CC:
-
Compound C
- CH:
-
Cholesterol–methyl-β-cyclodextrin
- CH + M:
-
Cholesterol–methyl-β-cyclodextrin + metformin
- ff BSA:
-
Fat-free BSA
- FAS:
-
Fatty acid synthase
- H:
-
Hypercholesterolemic
- H+M:
-
Hypercholesterolemic + metformin
- GFAT-1:
-
Glutamine fructose-6-phosphate amidotransferase 1
- HF:
-
High-fat
- HF+M:
-
High Fat + metformin
- HBP:
-
Hexosamine biosynthetic pathway
- HMGCoAS1:
-
3-hydroxy-3-methyl-glutaryl coenzyme A synthase 1
- ML:
-
Mother liver
- OGT:
-
O-Linked N-acetylglucosaminyltransferase
- OGA:
-
O-GlcNAcase
- PA:
-
Palmitic acid
- PA+M:
-
Palmitic acid + metformin
- PTM:
-
Post-translational modifications
- SREBP 1:
-
Sterol regulatory element-binding protein
- UDP-GlcNAc:
-
Uridine diphosphate N-acetylglucosamine
- 8W:
-
8 weeks
References
Akella NM, Ciraku L, Reginato MJ (2019) Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol 171(17):1–14. https://doi.org/10.1186/S12915-019-0671-3
Barbero-Becerra VJ, Santiago-Hernandez JA, Villegas-Lopez F et al (2012) Mechanisms involved in the protective effects of metformin against nonalcoholic fatty liver disease. Curr Med Chem 19:2918–2923. https://doi.org/10.2174/092986712800672094
Biwi J, Biot C, Guerardel Y et al (2018) The many ways by which O-GlcNAcylation may orchestrate the diversity of complex glycosylations. Mol 23:2858. https://doi.org/10.3390/MOLECULES23112858
Bullen JW, Balsbaugh JL, Chanda D et al (2014) Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and amp-activated protein kinase (AMPK) *. J Biol Chem 289:10592–10606. https://doi.org/10.1074/JBC.M113.523068
Cao Y, Liu X, Zhao J, Du M (2021) AMPKα1 regulates Idh2 transcription through H2B O-GlcNAcylation during brown adipogenesis. Acta Biochim Biophys Sin (Shanghai) 53:112–118. https://doi.org/10.1093/ABBS/GMAA136
Champattanachai V, Marchase RB, Chatham JC (2007) Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. 292:178–187. https://doi.org/10.1152/AJPCELL.00162.2006
Chatham JC, Marchase RB (2010) Protein O-GlcNAcylation: a critical regulator of the cellular response to stress. Curr Sign Trans Ther 5:49–59. https://doi.org/10.2174/157436210790226492
do Rosario VA, Chang C, Spencer J et al (2021) Anthocyanins attenuate vascular and inflammatory responses to a high fat high energy meal challenge in overweight older adults: a cross-over, randomized, double-blind clinical trial. Clin Nutr 40:879–889. https://doi.org/10.1016/J.CLNU.2020.09.041
Ducheix S, Magré J, Cariou B, Prieur X (2018) Chronic O-GlcNAcylation and diabetic cardiomyopathy: the bitterness of glucose. Front Endocrinol (Lausanne) 9:642. https://doi.org/10.3389/FENDO.2018.00642/BIBTEX
Emordi NG, Sunday I, Obimma OB (2020) ImageJ analysis of six different annealed temperatures of 0.17% C of HSLA steels. World J Eng Technol 08:617–629. https://doi.org/10.4236/WJET.2020.84043
Feng W-H, Bi Y, Li P et al (2019) Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: a randomized trial. J Diabetes Investig 10:399–407. https://doi.org/10.1111/jdi.12888
Folch J (1949) Brain diphosphoinositide, a new phosphatide having inositol metadiphosphate as a constituent. J Biol Chem 177:505–519. https://doi.org/10.1016/S0021-9258(18)56993-3
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509. https://doi.org/10.1016/s0021-9258(18)64849-5
Foot NC (2009) The Masson trichrome staining methods in routine laboratory use 8:101–110. https://doi.org/10.3109/10520293309116112
Gao M, Ma Y, Liu D (2015) High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One 10:e0119784. https://doi.org/10.1371/JOURNAL.PONE.0119784
Gélinas R, Dontaine J, Horman S et al (2018) AMP-activated protein kinase and O-GlcNAcylation, two partners tightly connected to regulate key cellular processes. Front Endocrinol (Lausanne) 9:519. https://doi.org/10.3389/FENDO.2018.00519/BIBTEX
Gélinas R, Mailleux F, Dontaine J et al (2018) AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat Commun 91(9):1–17. https://doi.org/10.1038/s41467-017-02795-4
Han C, Gu Y, Shan H et al (2017) O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat Commun 81(8):1–12. https://doi.org/10.1038/s41467-017-01654-6
Hanover JA, Krause MW, Love DC (2012) Linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol 135(13):312–321. https://doi.org/10.1038/nrm3334
Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O (2011) Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 80:825. https://doi.org/10.1146/ANNUREV-BIOCHEM-060608-102511
Ivancovsky-Wajcman D, Fliss-Isakov N, Webb M et al (2021) Ultra-processed food is associated with features of metabolic syndrome and non-alcoholic fatty liver disease. Liver Int 41:2635–2645. https://doi.org/10.1111/LIV.14996
Jagannath S, Chilkunda ND (2021) High cholesterol-supplemented diet during gestation and lactation alters liver glycosaminoglycans and associated lipoprotein receptors and results in fat accumulation in adulthood. Nutr Res 93:50–60. https://doi.org/10.1016/J.NUTRES.2021.07.002
Javitt NB (1990) Hep G2 cells as a resource for metabolic studies: lipoprotein, cholesterol, and bile acids. FASEB J 4:161–168. https://doi.org/10.1096/FASEBJ.4.2.2153592
Jiang M, Xu B, Li X et al (2018) O-GlcNAcylation promotes colorectal cancer metastasis via the miR-101-O-GlcNAc/EZH2 regulatory feedback circuit. Oncogene 383(38):301–316. https://doi.org/10.1038/s41388-018-0435-5
Kim MJ, Sim DY, Lee HM et al (2019) Hypolipogenic effect of shikimic acid via inhibition of MID1IP1 and phosphorylation of AMPK/ACC. Int J Mol Sci 20:582. https://doi.org/10.3390/IJMS20030582
Kitade H, Chen G, Ni Y, Ota T (2017) Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutr 9:387. https://doi.org/10.3390/NU9040387
Kuo YT, Lin TH, Chen WL, Lee HM (2012) Alpha-lipoic acid induces adipose triglyceride lipase expression and decreases intracellular lipid accumulation in HepG2 cells. Eur J Pharmacol 692:10–18. https://doi.org/10.1016/J.EJPHAR.2012.07.028
Li J, Liu M, Yu H et al (2018) Mangiferin improves hepatic lipid metabolism mainly through its metabolite-norathyriol by modulating SIRT-1/AMPK/SREBP-1c signaling. Front Pharmacol 9:201. https://doi.org/10.3389/FPHAR.2018.00201/BIBTEX
Lima VV, Giachini FR, Matsumoto T et al (2016) High-fat diet increases O-GlcNAc levels in cerebral arteries: a link to vascular dysfunction associated with hyperlipidaemia/obesity? Clin Sci 130:871–880. https://doi.org/10.1042/CS20150777
Lin MJ, Dai W, Scott MJ et al (2017) Metformin improves nonalcoholic fatty liver disease in obese mice via down-regulation of apolipoprotein A5 as part of the AMPK/LXRa signaling pathway. Oncotarget 8:108802–108809. https://doi.org/10.18632/oncotarget.22163
Liu F, Iqbal K, Grundke-Iqbal I et al (2004) O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci U S A 101:10804–10809. https://doi.org/10.1073/PNAS.0400348101
Lozano I, Van Der Werf R, Bietiger W et al (2016) High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr Metab 13:1–13. https://doi.org/10.1186/S12986-016-0074-1/FIGURES/3
Machacek M, Saunders H, Zhang Z et al (2019) Elevated O-GlcNAcylation enhances pro-inflammatory Th17 function by altering the intracellular lipid microenvironment. J Biol Chem 294:8973–8990. https://doi.org/10.1074/JBC.RA119.008373
Mailleux F, Gélinas R, Beauloye C et al (2016) O-GlcNAcylation, enemy or ally during cardiac hypertrophy development? Biochim Biophys Acta - Mol Basis Dis 1862:2232–2243. https://doi.org/10.1016/J.BBADIS.2016.08.012
Makino A, Suarez J, Gawlowski T et al (2011) Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol - Regul Integr Comp Physiol 300:1296–1302. https://doi.org/10.1152/AJPREGU.00437.2010/ASSET/IMAGES/LARGE/ZH60051175570006.JPEG
Medford HM, Chatham JC, Marsh SA (2012) Chronic ingestion of a Western diet increases O-linked-β-N-acetylglucosamine (O-GlcNAc) protein modification in the rat heart. Life Sci 90:883–888. https://doi.org/10.1016/J.LFS.2012.04.030
Motomura W, Inoue M, Ohtake T et al (2006) Up-regulation of ADRP in fatty liver in human and liver steatosis in mice fed with high fat diet. Biochem Biophys Res Commun 340:1111–1118. https://doi.org/10.1016/J.BBRC.2005.12.121
Ng YH, Okolo CA, Erickson JR et al (2021) Protein O-GlcNAcylation in the heart. Acta Physiol 233:e13696. https://doi.org/10.1111/APHA.13696
Nishimura K, Fujita Y, Ida S et al (2022) Glycaemia and body weight are regulated by sodium-glucose cotransporter 1 (SGLT1) expression via O-GlcNAcylation in the intestine. Mol Metab 59:101458. https://doi.org/10.1016/J.MOLMET.2022.101458
Olivier-Van Stichelen S, Dehennaut V, Buzy A et al (2014) O-GlcNAcylation stabilizes β-catenin through direct competition with phosphorylation at threonine 41. FASEB J 28:3325. https://doi.org/10.1096/FJ.13-243535
Pang Y, Xu X, Xiang X et al (2021) High fat activates O-GlcNAcylation and affects AMPK/ACC pathway to regulate lipid metabolism. Nutrients 13:1740. https://doi.org/10.3390/NU13061740/S1
Pinho TS, Correia SC, Perry G et al (2019) Diminished O-GlcNAcylation in Alzheimer’s disease is strongly correlated with mitochondrial anomalies. Biochim Biophys Acta - Mol Basis Dis 1865:2048–2059. https://doi.org/10.1016/J.BBADIS.2018.10.037
Porras D, Nistal E, Martínez-Flórez S et al (2017) Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med 102:188–202. https://doi.org/10.1016/J.FREERADBIOMED.2016.11.037
Prakoso D, Lim SY, Erickson JR et al (2022) Fine-tuning the cardiac O-GlcNAcylation regulatory enzymes governs the functional and structural phenotype of the diabetic heart. Cardiovasc Res 118:212–225. https://doi.org/10.1093/CVR/CVAB043
Rasband WS (1997-2016) ImageJ. U.S. National Institute of Health. References - Scientific Research Publishing, Bethesda, Maryland, USA https://www.scirp.org/reference/referencespapers.aspx?referenceid=2953546. Accessed 31 Mar 2022
Robarts DR, McGreal SR, Umbaugh DS et al (2022) Regulation of liver regeneration by hepatocyte O-GlcNAcylation in mice. Cmgh 13:1510–1529. https://doi.org/10.1016/j.jcmgh.2022.01.014
Sanchez-Rangel E, Inzucchi SE (2017) Metformin: clinical use in type 2 diabetes. Diabetologia 60:1586–1593. https://doi.org/10.1007/S00125-017-4336-X
Schweiger M, Romauch M, Schreiber R et al (2017) Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat Commun 81(8):1–15. https://doi.org/10.1038/ncomms14859
Shrikanth CB, Jagannath S, Chilkunda ND (2021) AMPK differentially alters sulphated glycosaminoglycans under normal and high glucose milieu in proximal tubular cells. J Biochem 169:75–86. https://doi.org/10.1093/JB/MVAA094
Steinberg GR, Carling D (2019) AMP-activated protein kinase: the current landscape for drug development. Nat Rev Drug Discov 187(18):527–551. https://doi.org/10.1038/s41573-019-0019-2
Thakur MS, Prapulla SG, Karanth NG (1988) Microscopic observation of Sudan Black B staining to monitor lipid production by microbes. J Chem Technol Biotechnol 42:129–134. https://doi.org/10.1002/JCTB.280420206
Tokubuchi I, Tajiri Y, Iwata S et al (2017) Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS One 12:e0171293. https://doi.org/10.1371/JOURNAL.PONE.0171293
Umapathi P, Mesubi OO, Banerjee PS et al (2021) Excessive O-GlcNAcylation causes heart failure and sudden death. Circulation 143:1687–1703. https://doi.org/10.1161/CIRCULATIONAHA.120.051911
Wang D, Wu J, Wang D et al (2021) Cisplatin enhances protein O-GlcNAcylation by altering the activity of OGT, OGA and AMPK in human non-small cell lung cancer cells. Int J Oncol 58:1–12. https://doi.org/10.3892/IJO.2021.5207/HTML
Wells L, Vosseller K, Hart GW (2003) A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci C 602(60):222–228. https://doi.org/10.1007/S000180300017
Wheatley EG, Albarran E, White CW et al (2019) Neuronal O-GlcNAcylation improves cognitive function in the aged mouse brain. Curr Biol 29:3359–3369.e4. https://doi.org/10.1016/J.CUB.2019.08.003
Wright JLN, Collins HE, Wende AR, Chatham JC (2017) O-GlcNAcylation and cardiovascular disease. Biochem Soc Trans 45:545–553. https://doi.org/10.1042/BST20160164
Zang M, Zuccollo A, Hou X et al (2004) AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem 279:47898–47905. https://doi.org/10.1074/jbc.M408149200
Zeidan Q, Hart GW (2010) The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways. J Cell Sci 123:13–22. https://doi.org/10.1242/JCS.053678
Zhang B, Li MD, Yin R et al (2019) O-GlcNAc transferase suppresses necroptosis and liver fibrosis. JCI Insight 4. https://doi.org/10.1172/JCI.INSIGHT.127709
Zhou J, Massey S, Story D, Li L (2018) Metformin: an old drug with new applications. Int J Mol Sci 19:2863. https://doi.org/10.3390/IJMS19102863
Zordoky BNM, Nagendran J, Pulinilkunnil T et al (2014) AMPK-dependent inhibitory phosphorylation of ACC is not essential for maintaining myocardial fatty acid oxidation. Circ Res 115:518–524. https://doi.org/10.1161/CIRCRESAHA.115.304538
Zuliani I, Lanzillotta C, Tramutola A et al (2021) High-fat diet leads to reduced protein O-GlcNAcylation and mitochondrial defects promoting the development of Alzheimer’s disease signatures. Int J Mol Sci 22:3746. https://doi.org/10.3390/IJMS22073746
Acknowledgements
SJ thanks Indian Council of Medical Research (ICMR) for the Senior Research Fellowship. The authors acknowledge the Director, CSIR-CFTRI, for keen interest.
Author information
Authors and Affiliations
Contributions
SJ: carried out bench work, analyzed data, and wrote manuscript. SHM: helped with experiments pertaining to animal studies. NCD: conceptualized, interpreted data, and wrote manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•O-GlcNAcylation is a highly context-dependent post-translational modification.
•High-cholesterol and high-fat diet individually resulted in decreased O-GlcNAcylation of liver proteins.
•Modulation of AMPK using pharmacological AMPK activator resulted in the restoration of O-GlcNAcylation levels when given as a preventive dose.
•Maternal hypercholesterolemia resulted in decreased O-GlcNAcylation in liver proteins of offspring in adulthood.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jagannath, S., Mallanna, S.H. & Nandini, C.D. Diet-inducing hypercholesterolemia show decreased O-GlcNAcylation of liver proteins through modulation of AMPK. J Physiol Biochem 80, 205–218 (2024). https://doi.org/10.1007/s13105-023-00997-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00997-7