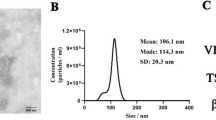
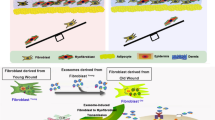
Abstract
Delayed wound healing is an urgent clinical issue. Cellular communication involving exosome-borne cargo such as miRNA is a critical mechanism involved in wound healing. This study isolated and identified human adipose tissue-derived exosomes (Exo-ATs). The specific effects of Exo-ATs on keratinocytes and fibroblasts were examined. Enriched miRNAs in Exo-ATs were analyzed, and miR-92a-3p was selected. The transfer of Exo-ATs-derived miR-92a-3p to keratinocytes and fibroblasts was verified. miR-92a-3p binding to LATS2 was examined and the dynamic effects of the miR-92a-3p/LATS2 axis were investigated. In a dorsal skin wound model, the in vivo effects of Exo-ATs on wound healing were examined. Exo-AT incubation increased keratinocytes and fibroblast proliferation, migration, and extracellular matrix (ECM) accumulation. miR-92a-3p, enriched in Exo-ATs, could be transferred to keratinocytes and fibroblasts, resulting in enhanced proliferation, migration, and ECM accumulation. Large tumor suppressor kinase 2 (LATS2) was a direct target of miR-92a-3p. miR-92a-3p inhibitor effects on keratinocytes and fibroblasts could be partially reversed by LATS2 knockdown. In a dorsal skin wound model, Exo-ATs accelerated wound healing through enhanced cell proliferation, collagen deposition, re-epithelialization, and YAP/TAZ activation. In conclusion, Exo-ATs improve skin wound healing by promoting keratinocyte and fibroblast migration and proliferation and collagen production by fibroblast, which could be partially eliminated by miR-92a inhibition through its downstream target LATS2 and the YAP/TAZ signaling.









Similar content being viewed by others
Data availability
The data generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Demidova-Rice TN, Hamblin MR, Herman IM (2012) Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 25:304–314
Blakytny R, Jude E (2006) The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 23:594–608
Pender LR, Frazier SK (2005) The relationship between dermal pressure ulcers, oxygenation and perfusion in mechanically ventilated patients. Intensive Crit Care Nurs 21:29–38
Werdin F, Tenenhaus M, Rennekampff HO (2008) Chronic wound care. Lancet 372:1860–1862
Tutuianu R, Rosca AM, Iacomi DM, Simionescu M, Titorencu I (2021) Human mesenchymal stromal cell-derived exosomes promote in vitro wound healing by modulating the biological properties of skin keratinocytes and fibroblasts and stimulating angiogenesis. Int J Mol Sci 22:6239
Zhang Y, Yu M, Dai M, Chen C, Tang Q, Jing W, Wang H, Tian W (2017) miR-450a-5p within rat adipose tissue exosome-like vesicles promotes adipogenic differentiation by targeting WISP2. J Cell Sci 130:1158–1168
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L (2016) Exosomes derived from human adipose mesenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep 6:32993
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420
Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A (2015) Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 6:715–731
Shim YH, Zhang RH (2017) Literature review to optimize the autologous fat transplantation procedure and recent technologies to improve graft viability and overall outcome: a systematic and retrospective analytic approach. Aesthetic Plast Surg 41:815–831
Connolly KD, Guschina IA, Yeung V, Clayton A, Draman MS, Von Ruhland C, Ludgate M, James PE, Rees DA (2015) Characterisation of adipocyte-derived extracellular vesicles released pre- and post-adipogenesis. J Extracell Vesicles 4:29159
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H et al (2015) HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 33:2158–2168
Hamam D, Ali D, Kassem M, Aldahmash A, Alajez NM (2015) microRNAs as regulators of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev 24:417–425
Shen S, Guo X, Yan H, Lu Y, Ji X, Li L, Liang T, Zhou D, Feng XH, Zhao JC et al (2015) A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res 25:997–1012
Zhu H, Luo H, Li Y, Zhou Y, Jiang Y, Chai J, Xiao X, You Y, Zuo X (2013) MicroRNA-21 in scleroderma fibrosis and its function in TGF-beta-regulated fibrosis-related genes expression. J Clin Immunol 33:1100–1109
Yang L, Zheng Z, Zhou Q, Bai X, Fan L, Yang C, Su L, Hu D (2017) miR-155 promotes cutaneous wound healing through enhanced keratinocytes migration by MMP-2. J Mol Histol 48:147–155
Pastar I, Khan AA, Stojadinovic O, Lebrun EA, Medina MC, Brem H, Kirsner RS, Jimenez JJ, Leslie C, Tomic-Canic M (2012) Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem 287:29324–29335
Liang X, Zhang L, Wang S, Han Q, Zhao RC (2016) Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci 129:2182–2189
Wang J, Zhang Y, Zhang N, Wang C, Herrler T, Li Q (2015) An updated review of mechanotransduction in skin disorders: transcriptional regulators, ion channels, and microRNAs. Cell Mol Life Sci 72:2091–2106
Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR et al (2011) Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 144:782–795
Lee DH, Park JO, Kim TS, Kim SK, Kim TH, Kim MC, Park GS, Kim JH, Kuninaka S, Olson EN et al (2016) LATS-YAP/TAZ controls lineage specification by regulating TGFbeta signaling and Hnf4alpha expression during liver development. Nat Commun 7:11961
Wang X, Ding X, Nan L, Wang Y, Wang J, Yan Z, Zhang W, Sun J, Zhu W, Ni B et al (2015) Investigation of the roles of exosomes in colorectal cancer liver metastasis. Oncol Rep 33:2445–2453
Luo Z, Lin J, Sun Y, Wang C, Chen J (2021) Bone marrow stromal cell-derived exosomes promote muscle healing following contusion through macrophage polarization. Stem Cells Dev 30:135–148
Peng Q, Zhang J, Zhou G (2019) Circulating exosomes regulate T-cell-mediated inflammatory response in oral lichen planus. J Oral Pathol Med 48:143–150
Ma S, Meng Z, Chen R, Guan KL (2019) The hippo pathway: biology and pathophysiology. Annu Rev Biochem 88:577–604
Lipson KE, Wong C, Teng Y, Spong S (2012) CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 5:S24
Cavey M, Rauzi M, Lenne PF, Lecuit T (2008) A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453:751–756
Zhan W, Tan SS, Lu F (2016) Adipose-derived stem cell delivery for adipose tissue engineering: current status and potential applications in a tissue engineering chamber model. Stem Cell Rev Rep 12:484–491
Chen B, Cai J, Wei Y, Jiang Z, Desjardins HE, Adams AE, Li S, Kao HK, Guo L (2019) Exosomes are comparable to source adipose stem cells in fat graft retention with up-regulating early inflammation and angiogenesis. Plast Reconstr Surg 144:816e–827e
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601
Bainbridge P (2013) Wound healing and the role of fibroblasts. J Wound Care 22(407–408):410–412
Werner S, Krieg T, Smola H (2007) Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 127:998–1008
Liu B, Li J, Cairns MJ (2014) Identifying miRNAs, targets and functions. Brief Bioinform 15:1–19
Lu TX, Rothenberg ME (2018) MicroRNA. J Allergy Clin Immunol 141:1202–1207
Hao Y, Chun A, Cheung K, Rashidi B, Yang X (2008) Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem 283:5496–5509
Zhang J, Smolen GA, Haber DA (2008) Negative regulation of YAP by LATS1 underscores evolutionary conservation of the drosophila hippo pathway. Cancer Res 68:2789–2794
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L et al (2007) Inactivation of YAP oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21:2747–2761
Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL (2008) TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 28:2426–2436
Pocaterra A, Romani P, Dupont S (2020) YAP/TAZ functions and their regulation at a glance. J Cell Sci 133:jcs230425
Reinke JM, Sorg H (2012) Wound repair and regeneration. Eur Surg Res 49:35–43
Hudson LG, Newkirk KM, Chandler HL, Choi C, Fossey SL, Parent AE, Kusewitt DF (2009) Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2). J Dermatol Sci 56:19–26
Sudol M, Bork P, Einbond A, Kastury K, Druck T, Negrini M, Huebner K, Lehman D (1995) Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem 270:14733–14741
Zhao B, Li L, Tumaneng K, Wang CY, Guan KL (2010) A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 24:72–85
Boopathy GTK, Hong W (2019) Role of hippo pathway-YAP/TAZ signaling in angiogenesis. Front Cell Dev Biol 7:49
Li CY, Hu J, Lu H, Lan J, Du W, Galicia N, Klein OD (2016) alphaE-catenin inhibits YAP/TAZ activity to regulate signalling centre formation during tooth development. Nat Commun 7:12133
Funding
This work has been supported by the National Natural Science Foundation of China (82800952), the Natural Science Foundation of Hunan Province, China (2021JJ30987), and the Key Research and Development Project of Hunan Province, China (2020sk2056).
Author information
Authors and Affiliations
Contributions
ZF S and JH X performed the experiments and wrote the main manuscript text. X W performed the experiments.YX Z coordinated the study. YJ W and YY L assisted with the experiments. JP Z and K L conceived the study, designed the experiments, and revised the paper. All the authors have reviewed and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All the procedures were approved by the Ethics Committee of Xiangya Stomatological Hospital of Central South University (no.20180006).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
1. Exo-AT derived from human adipose tissue was isolated and identified in this study.
2. Exo-AT enhances proliferation, migration, and ECM accumulation in keratinocytes and fibroblasts through miR-92a-3p transfer.
3. Exo-AT promotes wound healing by accelerating cell proliferation, collagen deposition, epithelialization, and YAP/TAZ activation.
4. MiR-92a-3p/LATS2 axis plays a key role in Exo-AT-mediated effects on keratinocytes and fibroblasts.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, Z., Xu, J., Wang, X. et al. Exosomes derived from adipose tissues accelerate fibroblasts and keratinocytes proliferation and cutaneous wound healing via miR-92a/Hippo-YAP axis. J Physiol Biochem 80, 189–204 (2024). https://doi.org/10.1007/s13105-023-00996-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00996-8