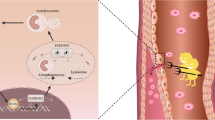
Abstract
Recently, trimethylamine N-oxide (TMAO) has been considered a risk factor for cardiovascular disease and has a proatherogenic effect. Many studies have found that TMAO is involved in plaque oxidative stress and lipid metabolism, but the specific mechanism is still unclear. In our study, meta-analysis and bioinformatic analysis were firstly conducted in the database, and found that the effect of high plasma TMAO levels on promoting atherosclerotic plaque may be related to the expression of key antioxidant genes nuclear factor erytheroid-derived-2-like 2 (NFE2L2/Nrf2) decreased. Next, we assessed the role of Nrf2-mediated signaling pathway in TMAO-treated foam cells. Our results showed that TMAO can inhibit the expression of Nrf2 and its downstream antioxidant response element such as heme oxygenase-1 (HO-1) and glutathione peroxidase4 (GPX4), resulting in increased production of reactive oxygen species and decreased activity of superoxide dismutase, promoting oxidative stress. And TMAO can also promote lipid accumulation in foam cells by inhibiting cholesterol efflux protein expression. In addition, upregulation of Nrf2 expression partially rescues TMAO-induced oxidative stress and reduces ATP-binding cassette A1 (ABCA1)–mediated lipid accumulation. Therefore, TMAO promotes oxidative stress and lipid accumulation in macrophage foam cells through the Nrf2/ABCA1 pathway, which may provide a potential mechanism for the proatherogenic effect of TMAO.







Similar content being viewed by others
Data availability
All data used to support the current study are available from the corresponding author on reasonable request. The links for the database included in the study are as follows: GEO database (http://www.ncbi.nlm.nih.gov/geo); Genecard database (http://www.genecards.org/).
Abbreviations
- ABCA1:
-
ATP-binding cassette A1
- ARE:
-
Antioxidant response element
- AS:
-
Atherosclerosis
- CVD:
-
Cardiovascular diseases
- GPX4:
-
Glutathione peroxidase4
- GEO:
-
Gene Expression Omnibus
- HO-1:
-
Heme oxygenase-1
- NFE2L2/Nrf2:
-
Nuclear factor erytheroid–derived-2-like 2
- Ox-LDL:
-
Oxidized low-density lipoprotein
- ROS:
-
Reactive oxygen species
- SOD:
-
Super oxide dismutase
- tBHQ:
-
Tert-butylhydroquinone
- TMAO:
-
Trimethylamine N-oxide
References
Alonso-Pineiro JA, Gonzalez-Rovira A, Sanchez-Gomar I et al (2021) Nrf2 and heme oxygenase-1 involvement in atherosclerosis related oxidative stress. Antioxidants (Basel) 9
Barajas B, Che N, Yin F et al (2011) NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol 1:58–66
Bekkering S, Quintin J, Joosten LA et al (2014) Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol 8:1731–1738
Bolen DW, Rose GD (2008) Structure and energetics of the hydrogen-bonded backbone in protein folding. Annu Rev Biochem:339–362
Brunt VE, Gioscia-Ryan RA, Richey JJ et al (2019) Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol 9:2361–2378
Brunt VE, Gioscia-Ryan RA, Casso AG et al (2020) Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 1:101–112
Brunt VE, Casso AG, Gioscia-Ryan RA et al (2021) Gut microbiome-derived metabolite trimethylamine N-oxide induces aortic stiffening and increases systolic blood pressure with aging in mice and humans. Hypertension 2:499–511
Chávez-Sánchez L, Garza-Reyes MG, Espinosa-Luna JE et al (2014) The role of TLR2, TLR4 and CD36 in macrophage activation and foam cell formation in response to oxLDL in humans. Hum Immunol 4:322–329
Chen ML, Zhu XH, Ran L et al (2017) Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J Am Heart Assoc 9
Chen S, Henderson A, Petriello MC et al (2019) Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab 6:1141–1151.e5
Chen YY, Ye ZS, Xia NG et al (2022) TMAO as a novel predictor of major adverse vascular events and recurrence in patients with large artery atherosclerotic ischemic stroke. Clin Appl Thromb Hemost:10760296221090503
Cuadrado A, Rojo AI, Wells G et al (2019) Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 4:295–317
da Costa RM, Rodrigues D, Pereira CA et al (2019) Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front Pharmacol 382
DeFilippis AP, Trainor PJ, Thanassoulis G et al (2022) Atherothrombotic factors and atherosclerotic cardiovascular events: the multi-ethnic study of atherosclerosis. Eur Heart J 10:971–981
Fan J, Watanabe T (2022) Atherosclerosis: known and unknown. Pathol Int 3:151–160
Folkersen L, Persson J, Ekstrand J et al (2012) Prediction of ischemic events on the basis of transcriptomic and genomic profiling in patients undergoing carotid endarterectomy. Mol Med 4:669–675
Freigang S, Ampenberger F, Spohn G et al (2011) Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol 7:2040–2051
Fukami K, Yamagishi S, Sakai K et al (2015) Oral L-carnitine supplementation increases trimethylamine-N-oxide but reduces markers of vascular injury in hemodialysis patients. J Cardiovasc Pharmacol 3:289–295
Gao J, Yan KT, Wang JX et al (2020) Gut microbial taxa as potential predictive biomarkers for acute coronary syndrome and post-STEMI cardiovascular events. Sci Rep 1:2639
Geng J, Yang C, Wang B et al (2018) Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed Pharmacother:941–947
Gonzalez-Correa C, Moleon J, Minano S et al (2021) Trimethylamine N-oxide promotes autoimmunity and a loss of vascular function in toll-like receptor 7-driven lupus mice. Antioxidants (Basel) 1
Guasti L, Galliazzo S, Molaro M et al (2021) TMAO as a biomarker of cardiovascular events: a systematic review and meta-analysis. Intern Emerg Med 1:201–207
Guo J, Ma J, Cai K et al (2022) Isoflavones from semen sojae preparatum improve atherosclerosis and oxidative stress by modulating Nrf2 signaling pathway through estrogen-like effects. Evid Based Complement Alternat Med:4242099
He LH, Gao JH, Yu XH et al (2020) Artesunate inhibits atherosclerosis by upregulating vascular smooth muscle cells-derived LPL expression via the KLF2/NRF2/TCF7L2 pathway. Eur J Pharmacol:173408
Hu CY, Lynch GC, Kokubo H et al (2010) Trimethylamine N-oxide influence on the backbone of proteins: an oligoglycine model. Proteins 3:695–704
Jiang X, Li Y, Wang W et al (2020) Nuclear factor erythroid 2 related factor 2 activator JC-5411 inhibits atherosclerosis through suppression of inflammation and regulation of lipid metabolism. Front Pharmacol:532568
Kattoor AJ, Pothineni NVK, Palagiri D et al (2017) Oxidative stress in atherosclerosis. Curr Atheroscler Rep 11:42
Khosravi M, Poursaleh A, Ghasempour G et al (2019) The effects of oxidative stress on the development of atherosclerosis. Biol Chem 6:711–732
Koeth RA, Wang Z, Levison BS et al (2013) Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 5:576–585
Li J, Meng Q, Fu Y et al (2021) Novel insights: dynamic foam cells derived from the macrophage in atherosclerosis. J Cell Physiol 9:6154–6167
Li L, Du Z, Rong B et al (2020) Foam cells promote atherosclerosis progression by releasing CXCL12. Biosci Rep 1
Liu J, Wang C, Li J et al (2021) Autophagy blockage promotes the pyroptosis of ox-LDL-treated macrophages by modulating the p62/Nrf2/ARE axis. J Physiol Biochem 3:419–429
Liu X, Xie Z, Sun M et al (2018) Plasma trimethylamine N-oxide is associated with vulnerable plaque characteristics in CAD patients as assessed by optical coherence tomography. Int J Cardiol:18–23
Liu Y, Dai M (2020) Trimethylamine N-oxide generated by the gut microbiota is associated with vascular inflammation: new insights into atherosclerosis. Mediators Inflamm:4634172
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 4:402–408
Lu Q, Tang SL, Liu XY et al (2013) Tertiary-butylhydroquinone upregulates expression of ATP-binding cassette transporter A1 via nuclear factor E2-related factor 2/heme oxygenase-1 signaling in THP-1 macrophage-derived foam cells. Circ J 9:2399–2408
Ma G, Pan B, Chen Y et al (2017) Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep 2
Mimura J, Itoh K (2015) Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic Biol Med (Pt B):221–232
Mohammadi A, Najar AG, Yaghoobi MM et al (2016) Trimethylamine-N-oxide treatment induces changes in the ATP-binding cassette transporter A1 and scavenger receptor A1 in murine macrophage J774A.1 cells. Inflammation 1:393–404
Mohammadi A, Vahabzadeh Z, Jamalzadeh S et al (2018) Trimethylamine-N-oxide, as a risk factor for atherosclerosis, induces stress in J774A.1 murine macrophages. Adv Med Sci 1:57–63
Oakley CI, Sanborn D, Rafie N et al (2018) The direct effect of trimethylamine N-oxide (TMAO) on cardiac muscle contractile mechanics. J Clin Transl Sci S1:30–30
Olek RA, Samulak JJ, Sawicka AK et al (2019) Increased trimethylamine N-oxide is not associated with oxidative stress markers in healthy aged women. Oxid Med Cell Longev:6247169
Peng Q, Liu H, Luo Z et al (2022) Effect of autophagy on ferroptosis in foam cells via Nrf2. Mol Cell Biochem 5:1597–1606
Randrianarisoa E, Lehn-Stefan A, Wang X et al (2016) Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep:26745
Rittig ST, Gaal L, Schubert MR et al (2021) Vascular impairment by trimethylamine N-oxide (TMAO) uptake and the first description of a TMAO transporter in endothelial cells. Circulation (Suppl_1)
Ruotsalainen AK, Inkala M, Partanen ME et al (2013) The absence of macrophage Nrf2 promotes early atherogenesis. Cardiovasc Res 1:107–115
Safran M, Dalah I, Alexander J et al (2010) GeneCards Version 3: the human gene integrator. Database (Oxford):baq020
Senthong V, Li XS, Hudec T et al (2016) Plasma trimethylamine N-oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol 22:2620–2628
Sheng Z, Tan Y, Liu C et al (2019) Relation of circulating trimethylamine N-oxide with coronary atherosclerotic burden in patients with ST-segment elevation myocardial infarction. Am J Cardiol 6:894–898
Shi W, Huang Y, Yang Z et al (2021) Reduction of TMAO level enhances the stability of carotid atherosclerotic plaque through promoting macrophage M2 polarization and efferocytosis. Biosci Rep 6
Steinke I, Ghanei N, Govindarajulu M et al (2020) Drug discovery and development of novel therapeutics for inhibiting TMAO in models of atherosclerosis and diabetes. Front Physiol:567899
Sun T, Zhang Y, Yin J et al (2021) Association of gut microbiota-dependent metabolite trimethylamine N-oxide with first ischemic stroke. J Atheroscler Thromb 4:320–328
Sun X, Jiao X, Ma Y et al (2016) Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun 1-2:63–70
Sykiotis GP, Bohmann D (2010) Stress-activated cap'n'collar transcription factors in aging and human disease. Sci Signal 112:re3
Tan Y, Sheng Z, Zhou P et al (2019) Plasma trimethylamine N-oxide as a novel biomarker for plaque rupture in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 1:e007281
Tang WH, Wang Z, Levison BS et al (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 17:1575–1584
Tang WHW, Li DY, Hazen SL (2019) Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol 3:137–154
Wang Z, Klipfell E, Bennett BJ et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 7341:57–63
Wang Z, Roberts AB, Buffa JA et al (2015) Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 7:1585–1595
Wu P, Chen J, Chen J et al (2020) Trimethylamine N-oxide promotes apoE(-/-) mice atherosclerosis by inducing vascular endothelial cell pyroptosis via the SDHB/ROS pathway. J Cell Physiol 10:6582–6591
Wu Y, Song F, Li Y et al (2021) Acacetin exerts antioxidant potential against atherosclerosis through Nrf2 pathway in apoE(-/-) Mice. J Cell Mol Med 1:521–534
Yang S, Dai H, Lu Y et al (2022) Trimethylamine N-oxide promotes cell proliferation and angiogenesis in colorectal cancer. Immunol Res:7043856
Zhang Y, Fu Y, Jia L et al (2022) TMT-based quantitative proteomic profiling of human monocyte-derived macrophages and foam cells. Proteome Sci 1:1
Zheng L, Zheng J, Xie Y et al (2019) Serum gut microbe-dependent trimethylamine N-oxide improves the prediction of future cardiovascular disease in a community-based general population. Atherosclerosis:126–131
Zhu W, Gregory JC, Org E et al (2016) Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 1:111–124
Funding
This study was supported by the grants from the National Natural Science Foundation of China (No. 81672084).
Author information
Authors and Affiliations
Contributions
First author: do experiments, write articles, participate in part of the experimental design. Second, third, and fourth authors: assist in completing the experiment. Corresponding author: participate in article revision, experimental design and supervision, financial support. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Research involving human participants and/or animals
This study does not involve human participants and/or animals.
Informed consent
All authors were informed and agreed to sign.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
•The proatherogenic effect of high plasma TMAO level might be associated with the differential expression of NFE2L2 decreased by meta-analysis and bioinformatic analysis.
•TMAO promotes oxidative stress by inhibiting Nrf2/ARE signaling axis in macrophage foam cell.
•TMAO induces lipid accumulation by inhibiting cholesterol efflux protein expression in foam cell which partially alleviated by upregulation of Nrf2.
Supplementary information
ESM 1
Supplementary Table 1 Characteristics of the Included Studies. Abbreviations: M, male; CVD, cardiovascular disease; STEMI, ST-segment elevation myocardial infarction; ACS, Acute coronary syndromes. Data of TMAO Levels are expressed as medians (interquartile ranges) or means ± standard deviations. (DOCX 61 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Z., Yu, X., Wang, C. et al. Trimethylamine N-oxide promotes oxidative stress and lipid accumulation in macrophage foam cells via the Nrf2/ABCA1 pathway. J Physiol Biochem 80, 67–79 (2024). https://doi.org/10.1007/s13105-023-00984-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00984-y