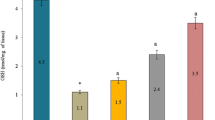
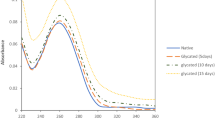
Abstract
In recent studies, we found that compounds derived from phenolic acids (CAFs) prevent the formation of the tubulin/aldose reductase complex and, consequently, may decrease the occurrence or delay the development of secondary pathologies associated with aldose reductase activation in diabetes mellitus. To verify this hypothesis, we determined the effect of CAFs on Na+,K+-ATPase tubulin-dependent activity in COS cells, ex vivo cataract formation in rat lenses and finally, to evaluate the antidiabetic effect of CAFs, diabetes mellitus was induced in Wistar rats, they were treated with different CAFs and four parameters were determinates: cataract formation, erythrocyte deformability, nephropathy and blood pressure. After confirming that CAFs are able to prevent the association between aldose reductase and tubulin, we found that treatment of diabetic rats with these compounds decreased membrane-associated acetylated tubulin, increased NKA activity, and thus reversed the development of four AR-activated complications of diabetes mellitus determined in this work. Based on these results, the existence of a new physiological mechanism is proposed, in which tubulin is a key regulator of aldose reductase activity. This mechanism can explain the incorrect functioning of aldose reductase and Na+,K+-ATPase, two key enzymes in the pathogenesis of diabetes mellitus. Moreover, we found that such alterations can be prevented by CAFs, which are able to dissociate tubulin/aldose reductase complex.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Brogard JM, Caro-Sampara F, Blickle JF (1992) Role of polyols in the development of diabetic complications. Value of aldose-reductase inhibitors. Rev Med Interne 13:69–79 doi:S0248-8663(05)80015-3
Cohen MP, Klepser H (1988) Glomerular Na+-K+-ATPase activity in acute and chronic diabetes and with aldose reductase inhibition. Diabetes 37:558–562. https://doi.org/10.2337/diab.37.5.558
Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA (2001) Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes 50:1927–1937. https://doi.org/10.2337/diabetes.50.8.1927
Raccah D, Dadoun F, Coste T, Vague P (1996) Decreased Na/K ATPase ouabain binding sites in red blood cells of patients with insulin-dependent diabetes and healthy north African control subjects: relationship with diabetic neuropathy. Horm Metab Res 28:128–132. https://doi.org/10.1055/s-2007-979144
Raccah D, Fabreguetts C, Azulay JP, Vague P (1996) Erythrocyte Na(+)-K(+)-ATPase activity, metabolic control, and neuropathy in IDDM patients. Diabetes Care 19:564–568. https://doi.org/10.2337/diacare.19.6.564
Vague P, Coste TC, Jannot MF, Raccah D, Tsimaratos M (2004) C-peptide, Na+,K(+)-ATPase, and diabetes. Exp Diabesity Res 5:37–50. https://doi.org/10.1080/15438600490424514
Sun W, Oates PJ, Coutcher JB, Gerhardinger C, Lorenzi M (2006) A selective aldose reductase inhibitor of a new structural class prevents or reverses early retinal abnormalities in experimental diabetic retinopathy. Diabetes 55:2757–2762 doi:55/10/2757
Casale CH, Alonso AD, Barra HS (2001) Brain plasma membrane Na+,K+-ATPase is inhibited by acetylated tubulin. Mol Cell Biochem 216:85–92. https://doi.org/10.1023/a:1011029125228
Casale CH, Previtali G, Barra HS (2003) Involvement of acetylated tubulin in the regulation of Na+,K+ -ATPase activity in cultured astrocytes. FEBS Lett 534:115–118 doi:S0014579302038024
Rivelli JF, Amaiden MR, Monesterolo NE, Previtali G, Santander VS, Fernandez A, Arce CA, Casale CH (2012) High glucose levels induce inhibition of Na,K-ATPase via stimulation of aldose reductase, formation of microtubules and formation of an acetylated tubulin/Na,K-ATPase complex. Int J Biochem Cell Biol 44:1203–1213. https://doi.org/10.1016/j.biocel.2012.04.011
Rivelli JF, Ochoa AL, Santander VS, Nigra A, Previtali G, Casale CH (2018) Regulation of aldose reductase activity by tubulin and phenolic acid derivates. Arch Biochem Biophys 654:19–26 doi:S0003-9861(18)30283-2
Salvador JM, Mata AM (1996) Purification of the synaptosomal plasma membrane (Ca(2+) + Mg(2+))-ATPase from pig brain. Biochem J 315(Pt 1):183–187. https://doi.org/10.1042/bj3150183
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 doi:0003-2697(76)90527-3
Brubaker AN, DeRuiter J, Whitmer WL (1986) Synthesis and rat lens aldose reductase inhibitory activity of some benzopyran-2-ones. J Med Chem 29:1094–1099. https://doi.org/10.1021/jm00156a031
Tabakoff B, Erwin VG (1970) Purification and characterization of a reduced nicotinamide adenine dinucleotide phosphate-linked aldehyde reductase from brain. J Biol Chem, vol 245, 1970/06/01 edn
Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE (2005) The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem 280:4617–4626
Cabrales P (2007) Effects of erythrocyte flexibility on microvascular perfusion and oxygenation during acute anemia. Am J Physiol Heart Circ Physiol 293:H1206–H1215 doi:00109.2007
Lee AY, Chung SK, Chung SS (1995) Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci U S A 92:2780–2784. https://doi.org/10.1073/pnas.92.7.2780
Reddy AB, Tammali R, Mishra R, Srivastava S, Srivastava SK, Ramana KV (2011) Aldose reductase deficiency protects sugar-induced lens opacification in rats. Chem Biol Interact 191:346–350. https://doi.org/10.1016/j.cbi.2011.02.028
Bareford D, Jennings PE, Stone PC, Baar S, Barnett AH, Stuart J (1986) Effects of hyperglycaemia and sorbitol accumulation on erythrocyte deformability in diabetes mellitus. J Clin Pathol 39:722–727. https://doi.org/10.1136/jcp.39.7.722
Chandra D, Jackson EB, Ramana KV, Kelley R, Srivastava SK, Bhatnagar A (2002) Nitric oxide prevents aldose reductase activation and sorbitol accumulation during diabetes. Diabetes 51:3095–3101. https://doi.org/10.2337/diabetes.51.10.3095
Kowluru R, Bitensky MW, Kowluru A, Dembo M, Keaton PA, Buican T (1989) Reversible sodium pump defect and swelling in the diabetic rat erythrocyte: effects on filterability and implications for microangiopathy. Proc Natl Acad Sci U S A 86:3327–3331. https://doi.org/10.1073/pnas.86.9.3327
Bashir SO (2019) Concomitant administration of resveratrol and insulin protects against diabetes mellitus type-1-induced renal damage and impaired function via an antioxidant-mediated mechanism and up-regulation of Na(+)/K(+)-ATPase. Arch Physiol Biochem 125:104–113. https://doi.org/10.1080/13813455.2018.1437752
ElGamal H, Munusamy S (2017) Aldose reductase as a drug target for treatment of diabetic nephropathy: promises and challenges. Protein Pept Lett 24:71–77. https://doi.org/10.2174/0929866523666161128153548
Gallicchio MA, Bach LA (2010) Advanced glycation end products inhibit Na+ K+ ATPase in proximal tubule epithelial cells: role of cytosolic phospholipase A2alpha and phosphatidylinositol 4-phosphate 5-kinase gamma. Biochim Biophys Acta 1803:919–930. https://doi.org/10.1016/j.bbamcr.2010.04.009
He J, Gao HX, Yang N, Zhu XD, Sun RB, Xie Y, Zeng CH, Zhang JW, Wang JK, Ding F, Aa JY, Wang GJ (2019) The aldose reductase inhibitor epalrestat exerts nephritic protection on diabetic nephropathy in db/db mice through metabolic modulation. Acta Pharmacol Sin 40:86–97. https://doi.org/10.1038/s41401-018-0043-5
Huang K, Liu W, Lan T, Xie X, Peng J, Huang J, Wang S, Shen X, Liu P, Huang H (2012) Berberine reduces fibronectin expression by suppressing the S1P-S1P2 receptor pathway in experimental diabetic nephropathy models. PLoS One 7:e43874. https://doi.org/10.1371/journal.pone.0043874
Iso K, Tada H, Kuboki K, Inokuchi T (2001) Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in Type 2 diabetic patients. J Diabetes Complicat 15:241–244. https://doi.org/10.1016/s1056-8727(01)00160-x
Xiu ZM, Wang LP, Fu J, Xu J, Liu L (2017) 1-Acetyl-5-phenyl-1H-pyrrol-3-ylacetate: An aldose reductase inhibitor for the treatment of diabetic nephropathy. Bioorg Med Chem Lett 27:4482–4487
Maheshwari R, Balaraman R, Sen AK, Shukla D, Seth A (2017) Effect of concomitant administration of coenzyme Q10 with sitagliptin on experimentally induced diabetic nephropathy in rats. Ren Fail 39:130–139. https://doi.org/10.1080/0886022X.2016.1254659
Hashimoto Y, Yamagishi S, Mizukami H, Yabe-Nishimura C, Lim SW, Kwon HM, Yagihashi S (2011) Polyol pathway and diabetic nephropathy revisited: early tubular cell changes and glomerulopathy in diabetic mice overexpressing human aldose reductase. J Diabetes Investig 2:111–122. https://doi.org/10.1111/j.2040-1124.2010.00071.x
Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, Tanabe K, Andres-Hernando A, Orlicky DJ, Finol E, Inaba S, Li N, Rivard CJ, Kosugi T, Sanchez-Lozada LG, Petrash JM, Sautin YY, Ejaz AA, Kitagawa W, Garcia GE, Bonthron DT, Asipu A, Diggle CP, Rodriguez-Iturbe B, Nakagawa T, Johnson RJ (2014) Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol 25:2526–2538. https://doi.org/10.1681/ASN.2013080901
Petrie JR, Guzik TJ, Touyz RM (2018) Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol 34:575–584 doi:S0828-282X(17)31214-X
Lee S, Lee MY, Nam JS, Kang S, Park JS, Shin S, Ahn CW, Kim KR (2015) Hemorheological approach for early detection of chronic kidney disease and diabetic nephropathy in type 2 diabetes. Diabetes Technol Ther 17:808–815. https://doi.org/10.1089/dia.2014.0295
Lee SB, Kim YS, Kim JH, Park K, Nam JS, Kang S, Park JS, Shin S, Ahn CW (2019) Use of RBC deformability index as an early marker of diabetic nephropathy. Clin Hemorheol Microcirc 72:75–84. https://doi.org/10.3233/CH-180434
Rebsomen L, Tsimaratos M (2005) Association of reduced red blood cell deformability and diabetic nephropathy. Kidney Int 67:2066; author reply 2066-2067. doi:S0085-2538(15)50693-5
Drel VR, Pacher P, Ali TK, Shin J, Julius U, El-Remessy AB, Obrosova IG (2008) Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative-nitrosative stress, glial activation, and apoptosis. Int J Mol Med 21:667–676
Hotta N, Akanuma Y, Kawamori R, Matsuoka K, Oka Y, Shichiri M, Toyota T, Nakashima M, Yoshimura I, Sakamoto N, Shigeta Y (2006) Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative Aldose Reductase Inhibitor-Diabetes Complications Trial. Diabetes Care 29:1538–1544 doi:29/7/1538
Casale CH, Previtali G, Serafino JJ, Arce CA, Barra HS (2005) Regulation of acetylated tubulin/Na+,K+-ATPase interaction by L-glutamate in non-neural cells: involvement of microtubules. Biochim Biophys Acta 1721:185–192 doi:S0304-4165(04)00287-9
Mantych GJ, Hageman GS, Devaskar SU (1993) Characterization of glucose transporter isoforms in the adult and developing human eye. Endocrinology 133:600–607. https://doi.org/10.1210/endo.133.2.8344201
Wood IS, Trayhurn P (2003) Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr 89:3–9. https://doi.org/10.1079/BJN2002763
Nigra AD, Monesterolo NE, Rivelli JF, Amaiden MR, Campetelli AN, Casale CH, Santander VS (2016) Alterations of hemorheological parameters and tubulin content in erythrocytes from diabetic subjects. Int J Biochem Cell Biol 74:109–120. https://doi.org/10.1016/j.biocel.2016.02.016
Amaiden MR, Santander VS, Monesterolo NE, Campetelli AN, Rivelli JF, Previtali G, Arce CA, Casale CH (2011) Tubulin pools in human erythrocytes: altered distribution in hypertensive patients affects Na+, K+-ATPase activity. Cell Mol Life Sci 68:1755–1768. https://doi.org/10.1007/s00018-010-0549-6
Monesterolo NE, Nigra AD, Campetelli AN, Santander VS, Rivelli JF, Arce CA, Casale CH (2015) PMCA activity and membrane tubulin affect deformability of erythrocytes from normal and hypertensive human subjects. Biochim Biophys Acta 1848:2813–2820. https://doi.org/10.1016/j.bbamem.2015.08.011
Amaiden MR, Monesterolo NE, Santander VS, Campetelli AN, Arce CA, Pie J, Hope SI, Vatta MS, Casale CH (2012) Involvement of membrane tubulin in erythrocyte deformability and blood pressure. J Hypertens 30:1414–1422. https://doi.org/10.1097/HJH.0b013e328353b19a
Amaiden MR, Santander VS, Monesterolo NE, Nigra AD, Rivelli JF, Campetelli AN, Pie J, Casale CH (2015) Effects of detyrosinated tubulin on Na+,K+-ATPase activity and erythrocyte function in hypertensive subjects. FEBS Lett 589:364–373. https://doi.org/10.1016/j.febslet.2014.12.022
Matsushima H, David LL, Hiraoka T, Clark JI (1997) Loss of cytoskeletal proteins and lens cell opacification in the selenite cataract model. Exp Eye Res 64:387–395 doi:S0014-4835(96)90220-1
Ghezzi C, Loo DDF, Wright EM (2018) Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 61:2087–2097. https://doi.org/10.1007/s00125-018-4656-5
Derosa G, Maffioli P (2018) Ertugliflozin: a sodium-glucose cotransporter-2 (SGLT-2) inhibitor for glycemic control in type 2 diabetes. Ther Clin Risk Manag 14:1637–1640. https://doi.org/10.2147/TCRM.S137068
Funding
The study was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica de la Secretaría de Ciencia y Tecnología del Ministerio de Cultura y Educación, as part of the Programa de Modernización Tecnológica (PICT-2018-03206), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto. We want to thank Florencia Sgarlatta for English editing.
Author information
Authors and Affiliations
Contributions
All the authors participated substantially in the conception and design of the work, the acquisition, analysis and interpretation of data, as well as in drafting the article or revising it critically to agree on the version to be published. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Research involving human participants and/or animals
All the experimental procedures were carried out in accordance with the guidelines approved by the Bioethics Committee of the National University of Río Cuarto (Coedi).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Treatment of cultured cells with CAFs prevented the formation of the Na+,K+-ATPase/Tub complex and restored NKA activity.
• In vivo treatment of rats with CAFs prevented the development of diabetic complications.
• These results provide the basis for a new approach to regulating aldose reductase activity and thus for designing novel treatment options for diabetes.
Supplementary Information
ESM 1
(DOCX 35 kb)
Rights and permissions
About this article
Cite this article
Rivelli Antonelli, J.F., Santander, V.S., Nigra, A.D. et al. Prevention of tubulin/aldose reductase association delays the development of pathological complications in diabetic rats. J Physiol Biochem 77, 565–576 (2021). https://doi.org/10.1007/s13105-021-00820-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-021-00820-1