Abstract
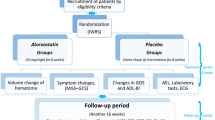
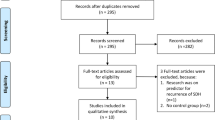
The objective of this study is to explore whether craniocervical manual lymphatic drainage (cMLD) can promote hematoma absorption and increase the efficiency of atorvastatin-based conservative treatment in chronic subdural hematoma (CSDH) patients. All CSDH patients treated with atorvastatin-based therapy between October 2020 and February 2022 in our department were retrospectively screened for enrollment. The patients were divided into the control and cMLD groups according to whether cMLD was performed. Head CT or MR images in both groups were obtained before the treatment and 2 weeks and 4 weeks after the treatment. MR images of the deep cervical lymphatic nodes (dCLNs) in 23 patients were obtained in the cMLD group before and approximately 2 weeks after treatment. The volumes of the dCLNs and hematoma were calculated. The primary outcomes are the differences in hematoma volume reduction after 4 weeks of treatment. The secondary outcomes were (1) the differences in hematoma volume reduction between the patients in these two groups in the 2nd week, (2) the dCLN volume change in the cMLD group before and after 2 weeks of treatment, and (3) the percentage of patients who transitioned to surgery because of failure to the conservative treatment. A total of 106 consecutive patients were enrolled in this study for analysis; 54 patients received atorvastatin-based treatment (control group), and 52 were treated with both atorvastatin-based treatment and cMLD (cMLD group). At baseline, the mean hematoma volume was 76.53 ± 42.97 ml in the control group and 88.57 ± 49.01 ml in the cMLD group (p = 0.181). In the 4th week, the absolute number of hematoma reductions (20.79 ± 34.73 ml vs. 37.28 ± 28.24 ml, p = 0.009) and percentage of hematoma reductions (22.58% ± 60.01% vs. 46.43% ± 30.12%, p = 0.012) in the cMLD group were greater than those in the control group. After 2 weeks of treatment, the absolute number of hematoma reductions showed no difference in the two groups, while the percentage of hematoma reduction was higher in the cMLD group (18.18% ± 24.61% vs. 2.08% ± 25.72%, p = 0.001). One patient in cMLD and 8 patients in the control group were transitioned to receive surgical treatment. The dCLN volumes in 23 experimental patients increased significantly after 2 weeks of treatment in the cMLD group (p = 0.032). There were no severe side effects that needed to be reported. Combined with atorvastatin-based therapy, cMLD can promote hematoma absorption and decrease the surgery rate, which provides a new therapeutic strategy for CSDH.




Similar content being viewed by others
Data Availability
The supporting data of this study are available on request from the corresponding author.
References
Miah I P, Herklots M, Roks G, et al. Dexamethasone therapy in symptomatic chronic subdural hematoma (DECSA–R): a retrospective evaluation of initial corticosteroid therapy versus primary surgery[J]. J Neurotrauma. 2020;37(2):366–372.
Gaist D, Garcia Rodriguez LA, Hellfritzsch M, et al. Association of antithrombotic drug use with subdural hematoma risk. JAMA. 2017;317(8):836–46.
Jiang R, Zhao S, Wang R, et al. Safety and efficacy of atorvastatin for chronic subdural hematoma in Chinese patients: a randomized clinicaltrial. JAMA Neurol. 2018;75(11):1338–46.
Rauhala M, Luoto TM, Huhtala H, et al. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg. 2019:1–11.
Iihara K, Saito N, Suzuki M, et al. The Japan Neurosurgical Database: statistics update 2018 and 2019. Neurol Med Chir (Tokyo). 2021;61(12):675–710.
Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015;123(5):1209–15.
Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol. 2014;10(10):570–8.
Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. 2011;114(1):72–6.
Rohde V, Graf G, Hassler W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: a retrospective analysis of 376 patients. Neurosurg Rev. 2002;25(1–2):89–94.
Thotakura AK, Marabathina NR. The role of medical treatment in chronic subdural hematoma. Asian J Neurosurg. 2018;13(4):976–83.
Holl DC, Volovici V, Dirven CMF, et al. Corticosteroid treatment compared with surgery in chronic subdural hematoma: a systematic review and meta-analysis. Acta Neurochir (Wien). 2019;161(6):1231–42.
Hutchinson PJ, Edlmann E, Bulters D, et al. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med. 2020;383(27):2616–27.
Poulsen FR, Munthe S, Soe M, Halle B. Perindopril and residual chronic subdural hematoma volumes six weeks after burr hole surgery: a randomized trial. Clin Neurol Neurosurg. 2014;123:4–8.
Bartek J Jr, Sjavik K, Schaible S, et al. The role of angiotensin-converting enzyme inhibitors in patients with chronic subdural hematoma: a Scandinavian population-based multicenter study. World Neurosurg. 2018;113:e555–60.
Holl DC, Volovici V, Dirven CMF, et al. Pathophysiology and nonsurgical treatment of chronic subdural hematoma: from past to present to future. World Neurosurg. 2018;116(402–411): e402.
Huang J, Li L, Zhang J, et al. Treatment of relapsed chronic subdural hematoma in four young children with atorvastatin and low-dose dexamethasone. Pharmacotherapy. 2019;39(7):783–9.
Wang D, Gao C, Xu X, et al. Treatment of chronic subdural hematoma with atorvastatin combined with low-dose dexamethasone: phase II randomized proof-of-concept clinical trial[J]. J Neurosurg. 2020;134(1):235–243.
Sun T, Yuan YK, Wu K, You C, Guan JW. Effects of postoperative atorvastatin use in elderly patients with chronic subdural hematoma. Eur Rev Med Pharmacol Sci. 2021;25(23):7211–7.
Chen S, Peng H, Shao X, et al. Prediction of risk factors for the evolution of traumatic subdural effusion into chronic subdural hematoma. Neuropsychiatr Dis Treat. 2020;16:943–8.
Chan DY, Chan DT, Sun TF, Ng SC, Wong GK, Poon WS. The use of atorvastatin for chronic subdural haematoma: a retrospective cohort comparison study(). Br J Neurosurg. 2017;31(1):72–7.
Zhang J, Chinese Society of Neurosurgery, Chinese Medical Association, et al. Expert consensus on drug treatment of chronic subdural hematoma[J]. Chin Neurosurg J. 2022;8(01):51–59.
Liu X, Gao C, Yuan J, et al. Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol Commun. 2020;8(1):16.
Gradalski T, Ochalek K, Kurpiewska J. Complex decongestive lymphatic therapy with or without Vodder II manual lymph drainage in more severe chronic postmastectomy upper limb lymphedema: a randomized noninferiority prospective study. J Pain Symptom Manage. 2015;50(6):750–7.
Bongi SM, Del Rosso A, Passalacqua M, Miccio S, Cerinic MM. Manual lymph drainage improving upper extremity edema and hand function in patients with systemic sclerosis in edematous phase. Arthritis Care Res (Hoboken). 2011;63(8):1134–41.
Vignes S, Simon L, Benoughidane B, Simon M, Fourgeaud C. Clinical and scintigraphic predictors of primary lower limb lymphedema-volume reduction during complete decongestive physical therapy. Phys Ther. 2020;100(5):766–72.
Jiang RC, Wang D, Zhao SG, et al. Atorvastatin combined with dexamethasone in chronic subdural haematoma (ATOCH II): study protocol for a randomized controlled trial. Trials. 2021;22(1):905.
Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. 2000;174(3):837–44.
Huang J, Tian Y, Song Y, et al. Effect of different factors on the short-term outcome of Chinese patients with primary chronic subdural hematoma at different age groups: a two-center retrospective study. Front Aging Neurosci. 2019;11:325.
Edlmann E, Holl D C, Lingsma H F, et al. Systematic review of current randomised control trials in chronic subdural haematoma and proposal for an international collaborative approach[J]. Acta neurochirurgica. 2020;162(4):763–776.
Huang J, Gao C, Dong J, et al. Drug treatment of chronic subdural hematoma[J]. Expert Opin Pharmacother. 2020;21(4):435–444.
Wang D, Li T, Tian Y, et al. Effects of atorvastatin on chronic subdural hematoma: a preliminary report from three medical centers. J Neurol Sci. 2014;336(1–2):237–42.
Qiu S, Zhuo W, Sun C, Su Z, Yan A, Shen L. Effects of atorvastatin on chronic subdural hematoma: a systematic review. Medicine (Baltimore). 2017;96(26): e7290.
Tang R, Shi J, Li X, et al. Effects of atorvastatin on surgical treatments of chronic subdural hematoma. World Neurosurg. 2018;117:e425–9.
Li T, Wang D, Tian Y, et al. Effects of atorvastatin on the inflammation regulation and elimination of subdural hematoma in rats. J Neurol Sci. 2014;341(1–2):88–96.
Nikolenko VN, Oganesyan MV, Vovkogon AD, et al. Current understanding of central nervous system drainage systems: implications in the context of neurodegenerative diseases. Curr Neuropharmacol. 2020;18(11):1054–63.
Ding XB, Wang XX, Xia DH, et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat Med. 2021;27(3):411–8.
Tsai HH, Hsieh YC, Lin JS, et al. Functional investigation of meningeal lymphatic system in experimental intracerebral hemorrhage. Stroke. 2022;53(3):987–98.
Yanev P, Poinsatte K, Hominick D, et al. Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab. 2020;40(2):263–75.
Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017;14(1):108.
Lopera C, Worsley PR, Bader DL, Fenlon D. Investigating the short-term effects of manual lymphatic drainage and compression garment therapies on lymphatic function using near-infrared imaging. Lymphat Res Biol. 2017;15(3):235–40.
Tacani PM, Franceschini JP, Tacani RE, et al. Retrospective study of the physical therapy modalities applied in head and neck l ymphedema treatment. Head Neck. 2016;38(2):301–8.
Liao SF, Li SH, Huang HY, et al. The efficacy of complex decongestive physiotherapy (CDP) and predictive factors of lymphedema severity and response to CDP in breast cancer-related lymphedema (BCRL). Breast. 2013;22(5):703–6.
Roth C, Stitz H, Roth C, et al. Craniocervical manual lymphatic drainage and its impact on intracranial pressure - a pilot study. Eur J Neurol. 2016;23(9):1441–6.
Ahn JH, Cho H, Kim JH, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572(7767):62–6.
Elmore SA. Histopathology of the lymph nodes. Toxicol Pathol. 2006;34(5):425–54.
Dorfman T, Neymark M, Begal J, Kluger Y. Surgical biopsy of pathologically enlarged lymph nodes: a reappraisal. Isr Med Assoc J. 2018;20(11):674–8.
Ying M, Pang BS. Three-dimensional ultrasound measurement of cervical lymph node volume. Br J Radiol. 2009;82(980):617–25.
Acknowledgements
We acknowledge Professor Jia He and her group in the Department of Health Statistics, Second Military Medical University, for the guidance of statistical analysis.
Funding
The authors are funded by the National Natural Science Foundation of China (via grant no. 82001323 to C Gao, grant no. 82071390 to R Jiang, and grant no. 82171359 to D Wang). They are also supported by the Beijing Tianjin Hebei Basic Research Cooperation Project (via grant no. 19JCZDJC64600(Z) to D Wang), the Tianjin Research Program of Application Foundation and Advanced Technology (via grant no. 19YFZCSY00650 to R Jiang), and the Clinical Study of Tianjin Medical University (2017kylc007 to R.J.).
Author information
Authors and Affiliations
Contributions
RJ, JW, JZ, and CG conceived and designed the study and provided funding. YW, CG, and JZ developed the methodology. JH, MN, XL, JY, DW, WJ, YT, SA, ZS, YF, JF, ML, SD, and DW collected the data and calculated the hematoma volume. CG and JW wrote the manuscript. JS and DW provided technical support. RJ and JZ reviewed and revised the manuscript and supervised the study. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval and Consent to Participate
This study was approved by the Ethical Committees of General Hospital of Tianjin Medical University in China. Informed consent was obtained from the participants or their legal representatives.
Human and Animal Ethics
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for Publication
All the authors have reviewed the manuscript and provided informed consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file3 (MP4 80506 kb) Video legend: The video shows the protocol of cMLD.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, C., Wei, Y., Zhang, X. et al. Craniocervical Manual Lymphatic Drainage Increases the Efficiency of Atorvastatin-Based Treatment of Chronic Subdural Hematoma. Transl. Stroke Res. 14, 667–677 (2023). https://doi.org/10.1007/s12975-022-01062-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01062-z