Abstract
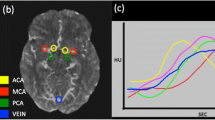
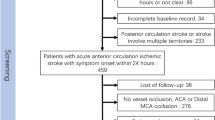
This study aimed to develop a supervised deep learning (DL) model for grading collateral status from dynamic susceptibility contrast magnetic resonance perfusion (DSC-MRP) images from patients with large vessel occlusion (LVO) acute ischemic stroke (AIS) and compare its performance against experts’ manual grading. Among consecutive LVO-AIS at three medical center sites, DSC-MRP data were processed to generate collateral flow maps consisting of arterial, capillary, and venous phases. With the use of expert readings as a reference, a DL model was developed to analyze collateral status with output classified into good and poor grades. The resulting model was externally validated in a later-collected population from one medical center site. The model was trained on 255 patients and externally validated on 72 patients. In the all-site internal validation population, DL grading of good collateral probability yielded a c statistic of 0.91; in the external validation population, the c statistic was 0.85. In the external validation population, there was moderate agreement between the experts’ grades and DL grades (kappa = 0.53, 95% CI = 0.32–0.73, p < 0.0001). Day 7 infarct growth volume was higher in DL-graded poor collateral group than good collateral group patients (median volume [26 mL vs. 6 mL], p = 0.01) in patients with successful reperfusion (modified treatment in cerebral infarction (mTICI) = 2b–3). In all patients with a 90-day modified Rankin Scale (mRS) score, there was a shift to more favorable outcomes in the good collateral group, with a common odds ratio of 2.99 (95% CI = 1.89–4.76, p < 0.0001). The DL-based collateral grading was in good agreement with expert manual grading in both development and validation populations. After exclusion of patients with large infarct volume, early reperfusion is more likely to benefit patients with the poor collateral flow, and the DL method has the potential to aid the assessment of collateral status.


Similar content being viewed by others
References
Albers GW, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:8708–18. https://doi.org/10.1056/NEJMoa1713973.
Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke. 2015;46(11):3302–9. https://doi.org/10.1161/STROKEAHA.115.010508.
Seo WK, et al. Predictors and functional outcomes of fast, intermediate, and slow progression among patients with acute ischemic stroke. Stroke. 2020;51(8):2553–7. https://doi.org/10.1161/STROKEAHA.120.030010.
Yu I, et al. Admission diffusion-weighted imaging lesion volume in patients with large vessel occlusion stroke and alberta stroke program early CT score of >/=6 points: serial computed tomography-magnetic resonance imaging collateral measurements. Stroke. 2019;50(11):3115–20. https://doi.org/10.1161/STROKEAHA.119.026229.
Kim BM, et al. Collateral status affects the onset-to-reperfusion time window for good outcome. J Neurol Neurosurg Psychiatry. 2018;89(9):903–9. https://doi.org/10.1136/jnnp-2017-317627.
Hassen W Ben, et al. Inter and intraobserver reliability for angiographic leptomeningeal collateral flow assessment by the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR scale). J Neurointerv Surg. 2019;11(4):338–41. https://doi.org/10.1136/neurintsurg-2018-014185.
Mair G, et al. Observer reliability of CT angiography in the assessment of acute ischaemic stroke: data from the Third International Stroke Trial. Neuroradiology. 2015;57(1):1–9. https://doi.org/10.1007/s00234-014-1441-0.
Berkhemer OA, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016;47(3):768–76. https://doi.org/10.1161/STROKEAHA.115.011788.
Kim SJ, et al. A novel magnetic resonance imaging approach to collateral flow imaging in ischemic stroke. Ann Neurol. 2014;76(3):356–69. https://doi.org/10.1002/ana.24211.
Jansen IGH, et al. Comparison of CTA- and DSA-based collateral flow assessment in patients with anterior circulation stroke. AJNR Am J Neuroradiol. 2016;37(11):2037–42. https://doi.org/10.3174/ajnr.A4878.
Seners P, et al. Better collaterals are independently associated with post-thrombolysis recanalization before thrombectomy. Stroke. 2019;50(4):867–72. https://doi.org/10.1161/STROKEAHA.118.022815.
Higashida RT, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109–37. https://doi.org/10.1161/01.STR.0000082721.62796.09.
Chollet F. Keras the python deep learning library. Astrophys Sour Code Libr. 2018;34:8.
Pedregosa F, et al. Scikit-learn: machine learning in Python. J Machine Lear Res. 2011;12:2825–30.
Vagal A, et al. Automated CT perfusion imaging for acute ischemic stroke: pearls and pitfalls for real-world use. Neurology. 2019;93(20):888–98. https://doi.org/10.1212/WNL.0000000000008481.
Demeestere J, Wouters A, Christensen S, Lemmens R, Lansberg MG. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51(3):1017–24. https://doi.org/10.1161/STROKEAHA.119.028337.
Nael K, et al. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: pushing the boundaries. Stroke. 2014;45(7):1985–91. https://doi.org/10.1161/STROKEAHA.114.005305.
You S-H, Kim B, Kim BK, Park SE. Fast MRI in acute ischemic stroke: applications of MRI acceleration techniques for MR-based comprehensive stroke imaging. Investigative Magnetic Resonance Imaging. 2021;25(2):81–92.
McKinley R, et al. Fully automated stroke tissue estimation using random forest classifiers (FASTER). J Cereb Blood Flow Metab. 2017;37(8):2728–41. https://doi.org/10.1177/0271678X16674221.
Nielsen A, Hansen MB, Tietze A, Mouridsen K. Prediction of tissue outcome and assessment of treatment effect in acute ischemic stroke using deep learning. Stroke. 2018;49(6):1394–401. https://doi.org/10.1161/STROKEAHA.117.019740.
Kim YC, et al. Novel estimation of penumbra zone based on infarct growth using machine learning techniques in acute ischemic stroke. J Clin Med. 2020;9:6. https://doi.org/10.3390/jcm9061977.
Brugnara G, et al. Multimodal predictive modeling of endovascular treatment outcome for acute ischemic stroke using machine-learning. Stroke. 2020;51(12):3541–51. https://doi.org/10.1161/STROKEAHA.120.030287.
To MNN, Kim HJ, Roh HG, Cho YS, Kwak JT. Deep regression neural networks for collateral imaging from dynamic susceptibility contrast-enhanced magnetic resonance perfusion in acute ischemic stroke. Int J Comput Assist Radiol Surg. 2020;15(1):151–62. https://doi.org/10.1007/s11548-019-02060-7.
Leng X, et al. Impact of collateral status on successful revascularization in endovascular treatment: a systematic review and meta-analysis. Cerebrovasc Dis. 2016;41(1–2):27–34. https://doi.org/10.1159/000441803.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, YC., Chung, JW., Bang, O.Y. et al. A Deep Learning-Based Automatic Collateral Assessment in Patients with Acute Ischemic Stroke. Transl. Stroke Res. 14, 66–72 (2023). https://doi.org/10.1007/s12975-022-01036-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01036-1