Abstract
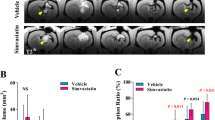
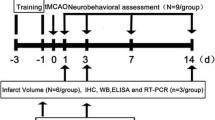
Intraventricular hemorrhage (IVH) is a subtype of intracerebral hemorrhage (ICH) with high morbidity and mortality. Posthemorrhagic hydrocephalus (PHH) is a common and major complication that affects prognosis, but the mechanism is still unclear. Inflammation and fibrosis have been well established as the major causes of PHH after IVH. In this study, we aimed to investigate the effects of metformin on IVH in adult male mice and further explored the underlying molecular mechanisms of these effects. In the acute phase, metformin treatment exerted dose-dependent neuroprotective effects by reducing periependymal apoptosis and neuronal degeneration and decreasing brain edema. Moreover, high-dose metformin reduced inflammatory cell infiltration and the release of proinflammatory factors, thus protecting ependymal structure integrity and subependymal neurons. In the chronic phase, metformin administration improved neurocognitive function and reduced delayed hydrocephalus. Additionally, metformin significantly inhibited basal subarachnoid fibrosis and ependymal glial scarring. The ependymal structures partially restored. Mechanically, IVH reduced phospho-AMPK (p-AMPK) and SIRT1 expression and activated the phospho-NF-κB (p-NF-κB) inflammatory signaling pathway. However, metformin treatment increased AMPK/SIRT1 expression and lowered the protein expression of p-NF-κB and its downstream inflammation. Compound C and EX527 administration reversed the anti-inflammatory effect of metformin. In conclusion, metformin attenuated neuroinflammation and subsequent fibrosis after IVH by regulating AMPK /SIRT1/ NF-κB pathways, thereby reducing delayed hydrocephalus. Metformin may be a promising therapeutic agent to prevent delayed hydrocephalus following IVH.











Similar content being viewed by others
Abbreviations
- IVH :
-
Intraventricular hemorrhage
- PHH :
-
Posthemorrhagic hydrocephalus
- ICH :
-
Intracerebral hemorrhage
- GMH :
-
Germinal matrix hemorrhage
- ICP :
-
Intracranial pressure
- ETV :
-
Endoscopic third ventriculostomy
- CSF :
-
Cerebrospinal fluid
- CPe :
-
Choroid plexus epithelium
- SVZ :
-
Subventricular zone
- CNS :
-
Central nervous system
- AMPK :
-
AMP-activated protein kinase
- NF-κB :
-
Nuclear factor-κB
- MRI :
-
Magnetic resonance imaging
- TUNEL :
-
Terminal deoxynucleotidyl transferase mediated dUTP nick end labeling
- FJC :
-
Fluoro-Jade C
- HE :
-
Hematoxylin and eosin
- PBS :
-
Phosphate-buffered saline
- Iba-1 :
-
Ionized calcium bindingadaptor molecule-1
- GFAP :
-
Glial fibrillary acidic protein
- SEM :
-
Scanning electron microscopy
- PBST :
-
PBS plus Tween-2
References
Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603–11. https://doi.org/10.1016/S0140-6736(16)32410-2.
Garton T, Hua Y, Xiang J, Xi G, Keep RF. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin Ther Targets. 2017;21(12):1111–22. https://doi.org/10.1080/14728222.2017.1397628.
Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 2012;3(Suppl 1):25–38. https://doi.org/10.1007/s12975-012-0182-9.
Bu Y, Chen M, Gao T, Wang X, Li X, Gao F. Mechanisms of hydrocephalus after intraventricular haemorrhage in adults. Stroke Vasc Neurol. 2016;1(1):23–7. https://doi.org/10.1136/svn-2015-000003.
Cherian S, Whitelaw A, Thoresen M, Love S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 2004;14(3):305–11. https://doi.org/10.1111/j.1750-3639.2004.tb00069.x.
Hallevi H, Albright KC, Aronowski J, Barreto AD, Martin-Schild S, Khaja AM, et al. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology. 2008;70(11):848–52. https://doi.org/10.1212/01.wnl.0000304930.47751.75.
Hallevi H, Walker KC, Kasam M, Bornstein N, Grotta JC, Savitz SI. Inflammatory response to intraventricular hemorrhage: time course, magnitude and effect of t-PA. J Neurol Sci. 2012;315(1–2):93–5. https://doi.org/10.1016/j.jns.2011.11.019.
Del Bigio MR. Pathophysiologic consequences of hydrocephalus. Neurosurg Clin N Am. 2001;12(4):639–49, vii.
Stagno V, Navarrete EA, Mirone G, Esposito F. Management of hydrocephalus around the world. World Neurosurg. 2013;79(2 Suppl):S23 e17–20. https://doi.org/10.1016/j.wneu.2012.02.004.
Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC. Hydrocephalus in children. Lancet. 2016;387(10020):788–99. https://doi.org/10.1016/S0140-6736(15)60694-8.
Kulkarni AV, Riva-Cambrin J, Butler J, Browd SR, Drake JM, Holubkov R, et al. Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls: clinical article. J Neurosurg Pediatr. 2013;12(4):334–8. https://doi.org/10.3171/2013.7.PEDS12637.
Karimy JK, Reeves BC, Damisah E, Duy PQ, Antwi P, David W, et al. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat Rev Neurol. 2020;16(5):285–96. https://doi.org/10.1038/s41582-020-0321-y.
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S, et al. Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery. 2010;67(3):588–93. https://doi.org/10.1227/01.NEU.0000373199.79462.21.
Kulkarni AV, Schiff SJ, Mbabazi-Kabachelor E, Mugamba J, Ssenyonga P, Donnelly R, et al. Endoscopic Treatment versus Shunting for Infant Hydrocephalus in Uganda. N Engl J Med. 2017;377(25):2456–64. https://doi.org/10.1056/NEJMoa1707568.
Vardakis JC, Tully BJ, Ventikos Y. Exploring the efficacy of endoscopic ventriculostomy for hydrocephalus treatment via a multicompartmental poroelastic model of CSF transport: a computational perspective. PLoS ONE. 2013;8(12): e84577. https://doi.org/10.1371/journal.pone.0084577.
Raper D, Louveau A, Kipnis J. How Do Meningeal Lymphatic Vessels Drain the CNS? Trends Neurosci. 2016;39(9):581–6. https://doi.org/10.1016/j.tins.2016.07.001.
Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–24. https://doi.org/10.1016/S1474-4422(18)30318-1.
Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23(8):997–1003. https://doi.org/10.1038/nm.4361.
Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132(23):5329–39. https://doi.org/10.1242/dev.02153.
Bai B, Chen H. Metformin: A Novel Weapon Against Inflammation. Front Pharmacol. 2021;12: 622262. https://doi.org/10.3389/fphar.2021.622262.
Tao L, Li D, Liu H, Jiang F, Xu Y, Cao Y, et al. Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-kappaB and MAPK signaling pathway. Brain Res Bull. 2018;140:154–61. https://doi.org/10.1016/j.brainresbull.2018.04.008.
Qi B, Hu L, Zhu L, Shang L, Wang X, Liu N, et al. Metformin Attenuates Neurological Deficit after Intracerebral Hemorrhage by Inhibiting Apoptosis, Oxidative Stress and Neuroinflammation in Rats. Neurochem Res. 2017;42(10):2912–20. https://doi.org/10.1007/s11064-017-2322-9.
Saffari PM, Alijanpour S, Takzaree N, Sahebgharani M, Etemad-Moghadam S, Noorbakhsh F, et al. Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer’s disease model. Life Sci. 2020;255: 117861. https://doi.org/10.1016/j.lfs.2020.117861.
Paudel YN, Angelopoulou E, Piperi C, Shaikh MF, Othman I. Emerging neuroprotective effect of metformin in Parkinson’s disease: A molecular crosstalk. Pharmacol Res. 2020;152: 104593. https://doi.org/10.1016/j.phrs.2019.104593.
Benjanuwattra J, Apaijai N, Chunchai T, Kerdphoo S, Jaiwongkam T, Arunsak B, et al. Metformin preferentially provides neuroprotection following cardiac ischemia/reperfusion in non-diabetic rats. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10): 165893. https://doi.org/10.1016/j.bbadis.2020.165893.
Fan YY, Wang YJ, Guo J, Wu MN, Zhang MS, Niu BL, et al. Delayed metformin treatment improves functional recovery following traumatic brain injury via central AMPK-dependent brain tissue repair. Brain Res Bull. 2020;164:146–56. https://doi.org/10.1016/j.brainresbull.2020.08.021.
Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z, et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun. 2018;69:351–63. https://doi.org/10.1016/j.bbi.2017.12.009.
Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24(8):1121–7. https://doi.org/10.1038/s41591-018-0087-6.
Yi H, Huang C, Shi Y, Cao Q, Chen J, Chen XM, et al. Metformin Attenuates Renal Fibrosis in a Mouse Model of Adenine-Induced Renal Injury Through Inhibiting TGF-beta1 Signaling Pathways. Front Cell Dev Biol. 2021;9:603802. https://doi.org/10.3389/fcell.2021.603802.
Jia W, Bai T, Zeng J, Niu Z, Fan D, Xu X, et al. Combined Administration of Metformin and Atorvastatin Attenuates Diabetic Cardiomyopathy by Inhibiting Inflammation, Apoptosis, and Oxidative Stress in Type 2 Diabetic Mice. Front Cell Dev Biol. 2021;9: 634900. https://doi.org/10.3389/fcell.2021.634900.
Zhu W, Gao Y, Chang CF, Wan JR, Zhu SS, Wang J. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLoS ONE. 2014;9(5): e97423. https://doi.org/10.1371/journal.pone.0097423.
Liu SP, Huang L, Flores J, Ding Y, Li P, Peng J, et al. Secukinumab attenuates reactive astrogliosis via IL-17RA/(C/EBPbeta)/SIRT1 pathway in a rat model of germinal matrix hemorrhage. CNS Neurosci Ther. 2019;25(10):1151–61. https://doi.org/10.1111/cns.13144.
Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab. 2006;26(12):1527–37. https://doi.org/10.1038/sj.jcbfm.9600306.
Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–58. https://doi.org/10.1038/nprot.2006.116.
Feng Z, Tan Q, Tang J, Li L, Tao Y, Chen Y, et al. Intraventricular administration of urokinase as a novel therapeutic approach for communicating hydrocephalus. Transl Res. 2017;180:77-90 e2. https://doi.org/10.1016/j.trsl.2016.08.004.
Garton T, Keep RF, Wilkinson DA, Strahle JM, Hua Y, Garton HJ, et al. Intraventricular Hemorrhage: the Role of Blood Components in Secondary Injury and Hydrocephalus. Transl Stroke Res. 2016;7(6):447–51. https://doi.org/10.1007/s12975-016-0480-8.
Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab. 2014;34(3):489–94. https://doi.org/10.1038/jcbfm.2013.225.
Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, et al. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 2008;39(12):3378–88. https://doi.org/10.1161/STROKEAHA.107.510883.
Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab. 2014;34(6):1070–5. https://doi.org/10.1038/jcbfm.2014.56.
Botfield H, Gonzalez AM, Abdullah O, Skjolding AD, Berry M, McAllister JP 2nd, et al. Decorin prevents the development of juvenile communicating hydrocephalus. Brain. 2013;136(Pt 9):2842–58. https://doi.org/10.1093/brain/awt203.
Lummis NC, Sanchez-Pavon P, Kennedy G, Frantz AJ, Kihara Y, Blaho VA, et al. LPA1/3 overactivation induces neonatal posthemorrhagic hydrocephalus through ependymal loss and ciliary dysfunction. Sci Adv. 2019;5(10):eaax2011. https://doi.org/10.1126/sciadv.aax2011.
Takizawa K, Matsumae M, Sunohara S, Yatsushiro S, Kuroda K. Characterization of cardiac- and respiratory-driven cerebrospinal fluid motion based on asynchronous phase-contrast magnetic resonance imaging in volunteers. Fluids Barriers CNS. 2017;14(1):25. https://doi.org/10.1186/s12987-017-0074-1.
Faubel R, Westendorf C, Bodenschatz E, Eichele G. Cilia-based flow network in the brain ventricles. Science. 2016;353(6295):176–8. https://doi.org/10.1126/science.aae0450.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85. https://doi.org/10.1007/s00125-017-4342-z.
Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020;32(1):44-55 e6. https://doi.org/10.1016/j.cmet.2020.04.015.
Liu Y, Tang G, Li Y, Wang Y, Chen X, Gu X, et al. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J Neuroinflammation. 2014;11:177. https://doi.org/10.1186/s12974-014-0177-4.
Jiang S, Li T, Yang Z, Yi W, Di S, Sun Y, et al. AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res Rev. 2017;38:18–27. https://doi.org/10.1016/j.arr.2017.07.001.
Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M, et al. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res. 2010;87(3):504–13. https://doi.org/10.1093/cvr/cvq066.
Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62. https://doi.org/10.1038/nrm3311.
Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104(17):7217–22. https://doi.org/10.1073/pnas.0610068104.
Velagapudi R, El-Bakoush A, Lepiarz I, Ogunrinade F, Olajide OA. AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol Cell Biochem. 2017;435(1–2):149–62. https://doi.org/10.1007/s11010-017-3064-3.
O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–55. https://doi.org/10.1038/nature11862.
Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, et al. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28(40):9989–96. https://doi.org/10.1523/JNEUROSCI.3257-08.2008.
Zhang F, Wang S, Gan L, Vosler PS, Gao Y, Zigmond MJ, et al. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95(3):373–95. https://doi.org/10.1016/j.pneurobio.2011.09.001.
Peng Y, Jin J, Fan L, Xu H, He P, Li J, et al. Rolipram Attenuates Early Brain Injury Following Experimental Subarachnoid Hemorrhage in Rats: Possibly via Regulating the SIRT1/NF-kappaB Pathway. Neurochem Res. 2018;43(4):785–95. https://doi.org/10.1007/s11064-018-2480-4.
Zhang XS, Wu Q, Wu LY, Ye ZN, Jiang TW, Li W, et al. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016;7(10): e2416. https://doi.org/10.1038/cddis.2016.292.
Chen S, Zhao L, Sherchan P, Ding Y, Yu J, Nowrangi D, et al. Activation of melanocortin receptor 4 with RO27-3225 attenuates neuroinflammation through AMPK/JNK/p38 MAPK pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2018;15(1):106. https://doi.org/10.1186/s12974-018-1140-6.
Zhu J, Liu K, Huang K, Gu Y, Hu Y, Pan S, et al. Metformin Improves Neurologic Outcome Via AMP-Activated Protein Kinase-Mediated Autophagy Activation in a Rat Model of Cardiac Arrest and Resuscitation. J Am Heart Assoc. 2018;7(12):19. https://doi.org/10.1161/JAHA.117.008389.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, Y., Liu, C., Li, G. et al. Metformin Alleviates Delayed Hydrocephalus after Intraventricular Hemorrhage by Inhibiting Inflammation and Fibrosis. Transl. Stroke Res. 14, 364–382 (2023). https://doi.org/10.1007/s12975-022-01026-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01026-3