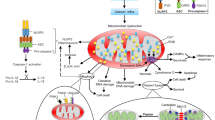
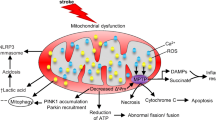
Abstract
Stroke is a debilitating condition which is also the second leading cause of death and disability worldwide. Despite the benefits and promises shown by numerous neuroprotective agents in animal stroke models, their clinical translation has not been a complete success. Hence, search for treatment options have directed researchers towards utilising stem cells. Mitochondria has a major involvement in the pathophysiology of stroke and a number of other conditions. Stem cells have shown the ability to transfer mitochondria to the damaged cells and to help revive cell energetics in the recipient cell. The present review discusses how stem cells could be employed to protect neurons and mitochondria in stroke and also the various mechanisms involved in neuroprotection.




Similar content being viewed by others
References
Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta (BBA)-Mol Basis Dise. 2017;1863(5):1066–77.
J-A K, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102(4):401–14.
Oyewole AO, Birch-Machin MA. Mitochondria-targeted antioxidants. FASEB J. 2015;29(12):4766–71.
Sherratt H. Mitochondria: structure and function. Rev Neurol. 1991;147(6–7):417–30.
Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80(5):780–7.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13.
Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–5.
Neri M, Fineschi V, Di Paolo M, Pomara C, Riezzo I, Turillazzi E, et al. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol. 2015;13(1):26–36.
Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120(4):713–35.
Wang X, Wang W, Li L, Perry G, H-g L, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2014;1842(8):1240–7.
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015;9:91.
Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6(2):109–20.
Kaur H, Sarmah D, Saraf J, Vats K, Kalia K, Borah A, et al. Noncoding RNAs in ischemic stroke: time to translate. Ann N Y Acad Sci. 2018;1421:19–36.
Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2010;1802(1):80–91.
Sarmah D, Agrawal V, Rane P, Bhute S, Watanabe M, Kalia K, et al. Mesenchymal stem cell therapy in ischemic stroke: a meta-analysis of preclinical studies. Clin Pharmacol Ther. 2018;103(6):990–98.
Sarmah D, Saraf J, Kaur H, Pravalika K, Tekade RK, Borah A, et al. Stroke management: an emerging role of nanotechnology. Micromachines. 2017;8(9):262.
Bhattacharya P, Pandey AK, Paul S, Patnaik R, Yavagal DR. Aquaporin-4 inhibition mediates piroxicam-induced neuroprotection against focal cerebral ischemia/reperfusion injury in rodents. PLoS One. 2013;8(9):e73481.
Sarmah D, Kaur H, Saraf J, Pravalika K, Goswami A, Kalia K, et al. Getting closer to an effective intervention of ischemic stroke: the big promise of stem cell. Transl Stroke Res. 2017:1–19.
d'Adesky N, Bhattacharya P, Schatz M, Perez-Pinzon M, Bramlett H, Raval A. Nicotine alters estrogen receptor-Beta-regulated Inflammasome activity and exacerbates ischemic brain damage in female rats. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19051330
Smajlović D. Strokes in young adults: epidemiology and prevention. Vasc Health Risk Manag. 2015;11:157.
Chapman SN, Mehndiratta P, Johansen MC, McMurry TL, Johnston KC, Southerland AM. Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vasc Health Risk Manag. 2014;10:75.
Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82(14):1277–86.
Meurer WJ, Barth BE, Gaddis G, Vilke GM, Lam SH. Rapid systematic review: intra-arterial thrombectomy (“clot retrieval”) for selected patients with acute ischemic stroke. J Emerg Med. 2017;52(2):255–61.
Pravalika K, Sarmah D, Kaur H, Wanve M, Saraf J, Kalia K, et al. Myeloperoxidase and neurological disorder: a crosstalk. ACS Chem Neurosci. 2018;9(3):421–30.
Bhattacharya P, Pandey AK, Paul S, Patnaik R. Melatonin renders neuroprotection by protein kinase C mediated aquaporin-4 inhibition in animal model of focal cerebral ischemia. Life Sci. 2014;100(2):97–109.
Bhattacharya P, Pandey AK, Paul S, Patnaik R. Neuroprotective potential of Piroxicam in cerebral ischemia: an in silico evaluation of the hypothesis to explore its therapeutic efficacy by inhibition of aquaporin-4 and acid sensing ion channel1a. Med Hypotheses. 2012;79(3):352–7.
Ginsberg MD. Adventures in the pathophysiology of brain ischemia: penumbra, gene expression, neuroprotection: the 2002 Thomas Willis lecture. Stroke. 2003;34(1):214–23.
Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol. 2009;61(6):321–30.
Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2012;1(3–4):185–99.
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361(16):1570–83.
Pandey AK, Shukla SC, Bhattacharya P, Patnaik R. A possible therapeutic potential of quercetin through inhibition of μ-calpain in hypoxia induced neuronal injury: a molecular dynamics simulation study. Neural Regen Res. 2016;11(8):1247–53.
Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–37.
Atchaneeyasakul K, Guada L, Ramdas K, Watanabe M, Bhattacharya P, Raval AP, et al. Large animal canine endovascular ischemic stroke models: a review. Brain Res Bull. 2016;127:134–40.
Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012;60(2):208–12.
Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M. Molecular mechanisms of ischemia–reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. 2013;47(1):9–23.
Gustafsson CM, Falkenberg M, Larsson N-G. Maintenance and expression of mammalian mitochondrial DNA. Annu Rev Biochem. 2016;85:133–60.
Kukat C, Davies KM, Wurm CA, Spåhr H, Bonekamp NA, Kühl I, et al. Cross-strand binding of TFAM to a single mtDNA molecule forms the mitochondrial nucleoid. Proc Natl Acad Sci. 2015;112(36):11288–93.
Torralba D, Baixauli F, Sánchez-Madrid F. Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front Cell Dev Biol. 2016;4(107):1–11.
Ghezzi D, Zeviani M. Assembly factors of human mitochondrial respiratory chain complexes: physiology and pathophysiology. Adv Exp Med Biol. 2012;748:65–106.
Dallner G, Sindelar PJ. Regulation of ubiquinone metabolism. Free Radic Biol Med. 2000;29(3–4):285–94.
Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87(7):1157–80.
Selivanov VA, Votyakova TV, Pivtoraiko VN, Zeak J, Sukhomlin T, Trucco M, et al. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput Biol. 2011;7(3):e1001115.
Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair. 2006;5(2):145–52.
Schieber M. Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R62.
Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:S210–S2.
Kussmaul L, Hirst J. The mechanism of superoxide production by NADH: ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci. 2006;103(20):7607–12.
Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH: ubiquinone oxidoreductase (complex I). J Biol Chem. 2004;279(38):39414–20.
Dong L-F, Jameson VJ, Tilly D, Cerny J, Mahdavian E, Marín-Hernández A, et al. Mitochondrial targeting of vitamin E succinate enhances its pro-apoptotic and anti-cancer activity via mitochondrial complex II. J Biol Chem. 2011;286(5):3717–28.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516.
Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, et al. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2010;1802(1):92–9.
Saito A, Hayashi T, Okuno S, Ferrand-Drake M, Chan PH. Interaction between XIAP and Smac/DIABLO in the mouse brain after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23(9):1010–9.
Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, et al. Apoptosis-inducing factor triggered by poly (ADP-ribose) polymerase and bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005;25(44):10262–72.
Zhou H, Wang J, Jiang J, Stavrovskaya IG, Li M, Li W, et al. N-acetyl-serotonin offers neuroprotection through inhibiting mitochondrial death pathways and autophagic activation in experimental models of ischemic injury. J Neurosci. 2014;34(8):2967–78.
Wang X, Figueroa BE, Stavrovskaya IG, Zhang Y, Sirianni AC, Zhu S, et al. Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury. Stroke. 2009;40(5):1877–85.
Martinvalet D, Zhu P, Lieberman J. Granzyme a induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity. 2005;22(3):355–70.
Philpott KL, McCarthy MJ, Klippel A, Rubin LL. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997;139(3):809–15.
Wang H-G, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284(5412):339–43.
Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6(5):513–9.
Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 2011;18(1):20–6.
Fannjiang Y, Cheng W-C, Lee SJ, Qi B, Pevsner J, McCaffery JM, et al. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004;18(22):2785–97.
Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574(1):33–9.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434(7029):113–8.
Barja G. Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res Rev. 2002;1(3):397–411.
Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, et al. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4(3):e76.
Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. FASEB J. 2000;14(12):1825–36.
Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357(6):562–71.
Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells antioxidant and antiapoptotic properties. J Biol Chem. 2001;276(7):4588–96.
James AM, Sharpley MS, Manas A-RB, Frerman FE, Hirst J, Smith RA, et al. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem. 2007;282(20):14708–18.
Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O'Sullivan JD, Fung V, et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Disord. 2010;25(11):1670–4.
Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30(7):1019–26.
Oyewole AO, Wilmot M-C, Fowler M, Birch-Machin MA. Comparing the effects of mitochondrial targeted and localized antioxidants with cellular antioxidants in human skin cells exposed to UVA and hydrogen peroxide. FASEB J. 2014;28(1):485–94.
Fang Y, Hu XH, Jia ZG, Xu MH, Guo ZY, Gao FH. Tiron protects against UVB-induced senescence-like characteristics in human dermal fibroblasts by the inhibition of superoxide anion production and glutathione depletion. Australas J Dermatol. 2012;53(3):172–80.
J Mailloux R. Application of mitochondria-targeted pharmaceuticals for the treatment of heart disease. Curr Pharm Des. 2016;22(31):4763–79.
Mao G, Kraus GA, Kim I, Spurlock ME, Bailey TB, Zhang Q, et al. A mitochondria-targeted vitamin E derivative decreases hepatic oxidative stress and inhibits fat deposition in mice–3. J Nutr. 2010;140(8):1425–31.
Yin X, Manczak M, Reddy PH. Mitochondria-targeted molecules MitoQ and SS31 reduce mutant huntingtin-induced mitochondrial toxicity and synaptic damage in Huntington's disease. Hum Mol Genet. 2016;25(9):1739–53.
Powell RD, Swet JH, Kennedy KL, Huynh TT, Murphy MP, Mckillop IH, et al. MitoQ modulates oxidative stress and decreases inflammation following hemorrhage. J Trauma Acute Care Surg. 2015;78(3):573–9.
Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, et al. Mitochondria-targeted antioxidants protect against amyloid-β toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20(s2):S609–S31.
Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2014;1842(8):1282–94.
Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17(13):1972–4.
Diener H-C, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II trials. Stroke. 2008;39(6):1751–8.
Ley JJ, Vigdorchik A, Belayev L, Zhao W, Busto R, Khoutorova L, et al. Stilbazulenyl nitrone, a second-generation azulenyl nitrone antioxidant, confers enduring neuroprotection in experimental focal cerebral ischemia in the rat: neurobehavior, histopathology, and pharmacokinetics. J Pharmacol Exp Ther. 2005;313(3):1090–100.
Becker DA, Ley JJ, Echegoyen L, Alvarado R. Stilbazulenyl nitrone (STAZN): a nitronyl-substituted hydrocarbon with the potency of classical phenolic chain-breaking antioxidants. J Am Chem Soc. 2002;124(17):4678–84.
Ley JJ, Belayev L, Saul I, Becker DA, Ginsberg MD. Neuroprotective effect of STAZN, a novel azulenyl nitrone antioxidant, in focal cerebral ischemia in rats: dose–response and therapeutic window. Brain Res. 2007;1180:101–10.
Reddy PH. Role of mitochondria in neurodegenerative diseases: mitochondria as a therapeutic target in Alzheimer’s disease. CNS Spectrums. 2009;14(S7):8–13.
Kuzmicic J, del Campo A, López-Crisosto C, Morales PE, Pennanen C, Bravo-Sagua R, et al. Mitochondrial dynamics: a potential new therapeutic target for heart failure. Rev Esp Cardiol (English Edition). 2011;64(10):916–23.
Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32(5):1183–94.
Godoy J, Allard C, Arrázola M, Zolezzi J, Inestrosa N. SIRT1 protects dendrites, mitochondria and synapses from Aβ oligomers in hippocampal neurons. J Alzheimers Dis Park. 2013;3(4):1–9.
Schenk S, McCurdy CE, Philp A, Chen MZ, Holliday MJ, Bandyopadhyay GK, et al. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest. 2011;121(11):4281–8.
Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444(7121):868–74.
Yu J, Auwerx J. The role of sirtuins in the control of metabolic homeostasis. Ann N Y Acad Sci. 2009;1173(1):E10–9.
Albiero M, Avogaro A, Fadini GP. A perspective on sirtuins in the metabolic syndrome. Metab Syndr Relat Disord. 2015;13(4):161–4.
Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9(4):371–8.
Khoury N, Koronowski KB, Young JI, Perez-Pinzon MA. The NAD+-dependent family of Sirtuins in cerebral ischemia and preconditioning. Antioxid Redox Signal. 2018;28(8):691–710.
Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159(3):993–1002.
Reddy PH. Inhibitors of mitochondrial fission as a therapeutic strategy for diseases with oxidative stress and mitochondrial dysfunction. J Alzheimers Dis. 2014;40(2):245–56.
Qi X, Qvit N, Su Y-C, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126(3):789–802.
Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14(2):193–204.
Meuer K, Suppanz I, Lingor P, Planchamp V, Göricke B, Fichtner L, et al. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ. 2007;14(4):651–61.
Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 2001;78(2):113–45.
Fredholm BB. Purinoceptors in the nervous system. Basic & Clinical Pharmacology & Toxicology. 1995;76(4):228–39.
Watts LT, Lloyd R, Garling RJ, Duong T. Stroke neuroprotection: targeting mitochondria. Brain Sci. 2013;3(2):540–60.
Williams M, Burnstock G. Purinergic neurotransmission and neuromodulation: a historical perspective. In: Jacobson, KA and Jarvis, MF, editors. Purinergic approaches in experimental therapeutics. New York: Wiley-Liss; 1997. p. 3–26.
Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84(4):476–88.
Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797.
Léon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y 1 receptor-null mice. J Clin Invest. 1999;104(12):1731–7.
Fabre J-E, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, et al. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y 1-deficient mice. Nat Med. 1999;5(10):1199–202.
Owens AP III, Mackman N. Tissue factor and thrombosis: the clot starts here. Thromb Haemost. 2010;104(03):432–9.
Zheng W, Watts LT, Holstein DM, Prajapati SI, Keller C, Grass EH, et al. Purinergic receptor stimulation reduces cytotoxic edema and brain infarcts in mouse induced by photothrombosis by energizing glial mitochondria. PLoS One. 2010;5(12):e14401.
Zheng W, Watts LT, Holstein DM, Wewer J, Lechleiter JD. P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J Cereb Blood Flow Metab. 2013;33(4):600–11.
Wu J, Holstein JD, Upadhyay G, Lin D-T, Conway S, Muller E, et al. Purinergic receptor-stimulated IP3-mediated Ca2+ release enhances neuroprotection by increasing astrocyte mitochondrial metabolism during aging. J Neurosci. 2007;27(24):6510–20.
Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol. 2012;96(1):32–45.
Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan L-J, et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011;286(18):16504–15.
Lin A-L, Poteet E, Du F, Gourav RC, Liu R, Wen Y, et al. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One. 2012;7(10):e46585.
Huang S, Du F, Shih Y-YI, Shen Q, Gonzalez-Lima F, Duong TQ. Methylene blue potentiates stimulus-evoked fMRI responses and cerebral oxygen consumption during normoxia and hypoxia. NeuroImage. 2013;72:237–42.
Holley AK, Bakthavatchalu V, Velez-Roman JM, St Clair DK. Manganese superoxide dismutase: guardian of the powerhouse. Int J Mol Sci. 2011;12(10):7114–62.
Holley AK, Dhar SK, Clair DKS. Manganese superoxide dismutase vs. p53: regulation of mitochondrial ROS. Mitochondrion. 2010;10(6):649–61.
Maier C, Hsieh L, Crandall T, Narasimhan P, Chan P. A new approach for the investigation of reperfusion-related brain injury. In: Portland press limited, vol. 34; 2006. p. 1366–9.
Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, et al. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18(20):8292–9.
Ivanović-Burmazović I. Reactivity of manganese superoxide dismutase mimics toward superoxide and nitric oxide: Selectivity versus cross-reactivity. In: Advances in inorganic chemistry. New York: Elsevier; 2012. p. 53–95.
Friedel FC, Lieb D, Ivanović-Burmazović I. Comparative studies on manganese-based SOD mimetics, including the phosphate effect, by using global spectral analysis. J Inorg Biochem. 2012;109:26–32.
Park W-C, Lim D-Y. Synthesis and SOD activity of manganese complexes of pentaaza macrocycles containing amino-and guanidino-auxiliary. Bull Kor Chem Soc. 2011;32(10):3787–9.
Shmonin A, Melnikova E, Galagudza M, Vlasov T. Characteristics of cerebral ischemia in major rat stroke models of middle cerebral artery ligation through craniectomy. Int J Stroke. 2014;9(6):793–801.
Huang HF, Guo F, Cao YZ, Shi W, Xia Q. Neuroprotection by manganese superoxide dismutase (MnSOD) mimics: antioxidant effect and oxidative stress regulation in acute experimental stroke. CNS Neurosci Ther. 2012;18(10):811–8.
Kelso GF, Maroz A, Cochemé HM, Logan A, Prime TA, Peskin AV, et al. A mitochondria-targeted macrocyclic Mn (II) superoxide dismutase mimetic. Chem Biol. 2012;19(10):1237–46.
Kondo T, Reaume AG, Huang T-T, Carlson E, Murakami K, Chen SF, et al. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17(11):4180–9.
Li M, Wang W, Mai H, Zhang X, Wang J, Gao Y, et al. Methazolamide improves neurological behavior by inhibition of neuron apoptosis in subarachnoid hemorrhage mice. Sci Rep. 2016;6:35055.
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22.
Borlongan CV. Age of PISCES: stem-cell clinical trials in stroke. Lancet. 2016;388(10046):736–8.
Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45(12):3618–24.
Hao L, Zou Z, Tian H, Zhang Y, Zhou H, Liu L. Stem cell-based therapies for ischemic stroke. Biomed Res Int. 2014;2014:1–17.
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535(7613):551–5.
Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci. 2006;103(5):1283–8.
Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–65.
Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Rehman R et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9):994–1010.
Cho YM, Kim JH, Kim M, Park SJ, Koh SH, Ahn HS, et al. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS One. 2012;7(3):e32778.
Lin H-Y, Liou C-W, Chen S-D, Hsu T-Y, Chuang J-H, Wang P-W, et al. Mitochondrial transfer from Wharton's jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion. 2015;22:31–44.
Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, Le Coz O, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29(5):812–24.
Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51(3):455–65.
Rogers RS, Bhattacharya J. When cells become organelle donors. Physiology. 2013;28(6):414–22.
Berridge MV, McConnell MJ, Grasso C, Bajzikova M, Kovarova J, Neuzil J. Horizontal transfer of mitochondria between mammalian cells: beyond co-culture approaches. Curr Opin Genet Dev. 2016;38:75–82.
Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10–8.
Han H, Hu J, Yan Q, Zhu J, Zhu Z, Chen Y, et al. Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol Med Rep. 2016;13(2):1517–24.
Plotnikov E, Khryapenkova T, Vasileva A, Marey M, Galkina S, Isaev N, et al. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med. 2008;12(5a):1622–31.
Mahrouf-Yorgov M, Augeul L, Da Silva CC, Jourdan M, Rigolet M, Manin S, et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24:1224–38.
Moschoi R, Imbert V, Nebout M, Chiche J, Mary D, Prebet T, et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood. 2016;128(2):253–64.
Bukoreshtliev NV, Wang X, Hodneland E, Gurke S, Barroso JF, Gerdes H-H. Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 2009;583(9):1481–8.
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes H-H. Nanotubular highways for intercellular organelle transport. Science. 2004;303(5660):1007–10.
He K, Shi X, Zhang X, Dang S, Ma X, Liu F, et al. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc Res. 2011;92(1):39–47.
Sun X, Wang Y, Zhang J, Tu J, Wang X, Su X, et al. Tunneling-nanotube direction determination in neurons and astrocytes. Cell Death Dis. 2012;3(12):e438.
Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, et al. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One. 2012;7(3):e33093.
Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13(5):328–35.
Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126(4):1139–43.
Jayaprakash AD, Benson EK, Gone S, Liang R, Shim J, Lambertini L, et al. Stable heteroplasmy at the single-cell level is facilitated by intercellular exchange of mtDNA. Nucleic Acids Res. 2015;43(4):2177–87.
Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, et al. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci. 2003;100(5):2397–402.
Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425(6961):968–73.
Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci. 2003;100(21):12313–8.
Vassilopoulos G, Wang P-R, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422(6934):901–4.
Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422(6934):897–901.
Nakajima A, Kurihara H, Yagita H, Okumura K, Nakano H. Mitochondrial extrusion through the cytoplasmic vacuoles during cell death. J Biol Chem. 2008;283(35):24128–35.
Lyamzaev KG, Nepryakhina OK, Saprunova VB, Bakeeva LE, Pletjushkina OY, Chernyak BV, et al. Novel mechanism of elimination of malfunctioning mitochondria (mitoptosis): formation of mitoptotic bodies and extrusion of mitochondrial material from the cell. Biochim Biophys Acta (BBA)-Bioenergetics. 2008;1777(7):817–25.
Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14(9):949–53.
Boudreau LH, Duchez A-C, Cloutier N, Soulet D, Martin N, Bollinger J, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase a 2 to promote inflammation. Blood. 2014;124(14):2173–83.
Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R, et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med. 2016;213:697–713. https://doi.org/10.1084/jem.20151876.
Babenko VA, Silachev DN, Popkov VA, Zorova LD, Pevzner IB, Plotnikov EY, et al. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules. 2018;23(3):687.
Funding
The authors acknowledge the Department of Science and Technology (DST), Govt. of India, for their financial support through grant (SB/YS/LS-196/2014), International Society for Neurochemistry (ISN) Return Home grant, Department of Pharmaceuticals, Ministry of Chemical and Fertilisers, Govt of India and National Institute of Pharmaceutical Education and Research (NIPER) Ahmedabad, Gandhinagar, India. The authors also want to express their thanks to the Director, NIPER Ahmedabad, for providing necessary support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sarmah, D., Kaur, H., Saraf, J. et al. Mitochondrial Dysfunction in Stroke: Implications of Stem Cell Therapy. Transl. Stroke Res. 10, 121–136 (2019). https://doi.org/10.1007/s12975-018-0642-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0642-y