Abstract
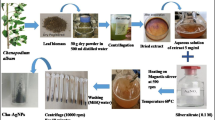
Sinigrin is one type of glucosinolates present in cruciferous plants which can be enzymatically hydrolyzed by myrosinase (MYR) to produce allyl isothiocyanate (AITC) with anti-neoplastic potency. This study demonstrates the anticancer activity of silver nanoparticles (AgNPs) green synthesized using sinigrin as a reducing and capping agent. The synthesized sinigrin-coated silver nanoparticles (SIN-AgNPs) characterized by analytical instruments (UV–Vis spectrometry, dynamic light scattering, transmission electron microscopy, and Raman spectroscopy) reveal a mean size of ⁓20 nm with good polydispersity, zeta-potential of -42 mV, and functional groups of sinigrin anchored on the surface of AgNPs. The cDNA of myrosinase was cloned in pcDNA3-eGFP plasmid and transfected to A549 lung adenocarcinoma cells to overexpress myrosinase. The average IC50 values of SIN-AgNPs against parental and myrosinase-expressed A549 cells using the MTT assay were determined to be 3 and 20 µg/ml, respectively. The apoptotic level of myrosinase-expressed A549 cells treated with SIN-AgNPs was further confirmed to be higher than the parental A549 cells.







Similar content being viewed by others
Data Availability
All data supporting the findings of this study are available within the paper.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2021). Cancer Statistics. CA: A Cancer Journal for Clinicians, 71(1), 7–33. https://doi.org/10.3322/CAAC.21654
Kayl, A. E., & Meyers, C. A. (2006). Side-Effects of Chemotherapy and Quality of Life in Ovarian and Breast Cancer Patients. Current Opinion in Obstetrics and Gynecology, 18(1), 24–28. https://doi.org/10.1097/01.GCO.0000192996.20040.24
Xu, W., Ye, C., Qing, X., Liu, S., Lv, X., Wang, W., Dong, X., Multi-Target, Z. Y., & Inhibitor, T. K. (2022). Nanoparticle Delivery Systems for Cancer Therapy. Materials Today Bio, 16, 100358. https://doi.org/10.1016/J.MTBIO.2022.100358
Niedzwiecki, A., Roomi, M. W., Kalinovsky, T., & Rath, M. (2016). Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients, 8(9), 552. https://doi.org/10.3390/NU8090552
Venkatadri, B., Shanparvish, E., Rameshkumar, M. R., Arasu, M. V., Al-Dhabi, N. A., Ponnusamy, V. K., & Agastian, P. (2020). Green Synthesis of Silver Nanoparticles Using Aqueous Rhizome Extract of Zingiber Officinale and Curcuma Longa: In-Vitro Anti-Cancer Potential on Human Colon Carcinoma HT-29 Cells. Saudi Journal of Biological Sciences, 27(11), 2980–2986. https://doi.org/10.1016/J.SJBS.2020.09.021
Sen, T., & Samanta, S. K. (2014). Medicinal Plants, Human Health and Biodiversity: A Broad Review. Advances in Biochemical Engineering/Biotechnology, 147, 59–110. https://doi.org/10.1007/10_2014_273/COVER
Maher, T., Raus, R. A., Daddiouaissa, D., Ahmad, F., Adzhar, N. S., Latif, E. S., Abdulhafiz, F., & Mohammed, A. (2021). Medicinal Plants with Anti-Leukemic Effects: A Review. Molecules, 26(9), 2741. https://doi.org/10.3390/MOLECULES26092741
Mazumder, A., Dwivedi, A., du Plessis, J., Mazumder, A., Dwivedi, A., & Du Plessis, J. (2016). Sinigrin and Its Therapeutic Benefits. Molecules, 21(4), 416. https://doi.org/10.3390/molecules21040416
Chang, P. Y., Tsai, F. J., Bau, D. T., Hsu, Y. M., Yang, J. Sing., Tu, M. G., & Lun, C. S. (2021). Potential Effects of Allyl Isothiocyanate on Inhibiting Cellular Proliferation and Inducing Apoptotic Pathway in Human Cisplatin-Resistant Oral Cancer Cells. Journal of the Formosan Medical Association, 120(1 Pt 2), 515–523. https://doi.org/10.1016/j.jfma.2020.06.025
Qin, G., Li, P., & Xue, Z. (2018). Effect of Allyl Isothiocyanate on the Viability and Apoptosis of the Human Cervical Cancer HeLa Cell Line in Vitro. Oncology Letters, 15(6), 8756–8760. https://doi.org/10.3892/ol.2018.8428
Liu, P., Behray, M., Wang, Q., Wang, W., Zhou, Z., Chao, Y., & Bao, Y. (2018). Anti-Cancer Activities of Allyl Isothiocyanate and Its Conjugated Silicon Quantum Dots. Scientific Reports, 8(1), 1084. https://doi.org/10.1038/s41598-018-19353-7
Núñez-Iglesias, M. J., Novío, S., García, C., Pérez-Muñuzuri, E., Soengas, P., Cartea, E., Velasco, P., & Freire-Garabal, M. (2019). Glucosinolate-Degradation Products as Co-Adjuvant Therapy on Prostate Cancer in Vitro. International Journal of Molecular Sciences, 20(20), 4977. https://doi.org/10.3390/ijms20204977
Lai, K. C., Lu, C. C., Tang, Y. J., Chiang, J. H., Kuo, D. H., Chen, F. A., Chen, I. L., & Yang, J. S. (2014). Allyl Isothiocyanate Inhibits Cell Metastasis through Suppression of the MAPK Pathways in Epidermal Growth Factor-Stimulated HT29 Human Colorectal Adenocarcinoma Cells. Oncology Reports, 31(1), 189–196. https://doi.org/10.3892/or.2013.2865
Sávio, A. L. V., da Silva, G. N., & Salvadori, D. M. F. (2015). Inhibition of Bladder Cancer Cell Proliferation by Allyl Isothiocyanate (Mustard Essential Oil). Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis, 771, 29–35. https://doi.org/10.1016/j.mrfmmm.2014.11.004
Geng, F., Tang, L., Li, Y., Yang, L., Choi, K. S., Kazim, A. L., & Zhang, Y. (2011). Allyl Isothiocyanate Arrests Cancer Cells in Mitosis, and Mitotic Arrest in Turn Leads to Apoptosis via Bcl-2 Protein Phosphorylation. Journal of Biological Chemistry, 286(37), 32259–32267. https://doi.org/10.1074/jbc.M111.278127
Savio, A. L. V., da Silva, G. N., Camargo, E. A., & de; Salvadori, D. M. F. (2014). Cell Cycle Kinetics, Apoptosis Rates, DNA Damage and TP53 Gene Expression in Bladder Cancer Cells Treated with Allyl Isothiocyanate (Mustard Essential Oil). Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis, 762(1), 40–46. https://doi.org/10.1016/j.mrfmmm.2014.02.006
Tsai, S. C., Huang, W. W., Huang, W. C., Lu, C. C., Chiang, J. H., Peng, S. F., Chung, J. G., Lin, Y. H., Hsu, Y. M., Amagaya, S., et al. (2012). ERK-Modulated Intrinsic Signaling and G2/M Phase Arrest Contribute to the Induction of Apoptotic Death by Allyl Isothiocyanate in MDA-MB-468 Human Breast Adenocarcinoma Cells. International Journal of Oncology, 41(6), 2065–2072. https://doi.org/10.3892/ijo.2012.1640
Ratan, Z. A., Haidere, M. F., Nurunnabi, M., Shahriar, S. M., Ahammad, A. J. S., Shim, Y. Y., Reaney, M. J. T., & Cho, J. Y. (2020). Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers, 12(4), 855. https://doi.org/10.3390/cancers12040855
Mathur, P., Jha, S., Ramteke, S., & Jain, N. K. (2018). Pharmaceutical Aspects of Silver Nanoparticles. Artificial Cells, Nanomedicine and Biotechnology, 46(sup1), 115–126. https://doi.org/10.1080/21691401.2017.1414825
Chugh, H., Sood, D., Chandra, I., Tomar, V., Dhawan, G., & Chandra, R. (2018). Role of Gold and Silver Nanoparticles in Cancer. Nano-Medicine, 46(sup1), 1210–1220. https://doi.org/10.1080/21691401.2018.1449118
Iravani, S., Korbekandi, H., Mirmohammadi, S. V., & Zolfaghari, B. (2014). Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Research in Pharmaceutical Sciences, 9(6), 385–406.
Nath, D., & Banerjee, P. (2013). Green Nanotechnology – A New Hope for Medical Biology. Environmental Toxicology and Pharmacology, 36(3), 997–1014. https://doi.org/10.1016/J.ETAP.2013.09.002
Park, Y. (2014). A New Paradigm Shift for the Green Synthesis of Antibacterial Silver Nanoparticles Utilizing Plant Extracts. Toxicology Research, 30(3), 169–178. https://doi.org/10.5487/TR.2014.30.3.169
Ovais, M., Khalil, A. T., Raza, A., Khan, M. A., Ahmad, I., Islam, N. U., Saravanan, M., Ubaid, M. F., Ali, M., & Shinwari, Z. K. (2016). Green Synthesis of Silver Nanoparticles via Plant Extracts: Beginning a New Era in Cancer Theranostics. Nanomedicine, 12(23), 3157–3177. https://doi.org/10.2217/nnm-2016-0279
Tarar, A., & Peng, C.-A. (2022). Enhancement of Antibacterial Activity of Sinigrin-Capped Silver Nanoparticles in Combination with Myrosinase. Journal of Environmental Chemical Engineering, 10(3), 107796. https://doi.org/10.1016/J.JECE.2022.107796
Kalishwaralal, K., BarathManiKanth, S., Pandian, S. R. K., Deepak, V., & Gurunathan, S. (2010). Silver Nanoparticles Impede the Biofilm Formation by Pseudomonas Aeruginosa and Staphylococcus Epidermidis. Colloids and Surfaces B: Biointerfaces, 79(2), 340–344. https://doi.org/10.1016/j.colsurfb.2010.04.014
Saxena, J., Sharma, P. K., Sharma, M. M., Singh, A. (2016). Process Optimization for Green Synthesis of Silver Nanoparticles by Sclerotinia Sclerotiorum MTCC 8785 and Evaluation of Its Antibacterial Properties. SpringerPlus 5 (861) https://doi.org/10.1186/s40064-016-2558-x.
Philip, D. (2010). Green Synthesis of Gold and Silver Nanoparticles Using Hibiscus Rosa Sinensis. Physica E: Low-Dimensional Systems and Nanostructures, 42(5), 1417–1424. https://doi.org/10.1016/j.physe.2009.11.081
Mousavi, S. M., Hashemi, S. A., Ghasemi, Y., Atapour, A., Amani, A. M., Savar Dashtaki, A., Babapoor, A., & Arjmand, O. (2018). Green Synthesis of Silver Nanoparticles toward Bio and Medical Applications: Review Study. Artificial Cells, Nanomedicine and Biotechnology, 46(sup3), S855–S872. https://doi.org/10.1080/21691401.2018.1517769
Pryshchepa, O., Pomastowski, P., & Buszewski, B. (2020). Silver Nanoparticles: Synthesis, Investigation Techniques, and Properties. Advances in Colloid and Interface Science, 284, 102246. https://doi.org/10.1016/J.CIS.2020.102246
Shao, J., Lin, M., Li, Y., Li, X., Liu, J., Liang, J., Yao, H. (2012) In Vivo Blood Glucose Quantification Using Raman Spectroscopy. PLoS One, 7 (10). https://doi.org/10.1371/JOURNAL.PONE.0048127.
Yang, X., Zhang, A. Y., Wheeler, D. A., Bond, T. C., Gu, C., & Li, Y. (2012). Direct Molecule-Specific Glucose Detection by Raman Spectroscopy Based on Photonic Crystal Fiber. Analytical and Bioanalytical Chemistry, 402(2), 687–691. https://doi.org/10.1007/S00216-011-5575-1
Ben Mabrouk, K., Kauffmann, T. H., Aroui, H., & Fontana, M. D. (2013). Raman Study of Cation Effect on Sulfate Vibration Modes in Solid State and in Aqueous Solutions. Journal of Raman Spectroscopy, 44(11), 1603–1608. https://doi.org/10.1002/jrs.4374
Kubitza, S., Vogt, D. S., Rammelkamp, K., Böttger, U., Frohmann, S., Hansen, P. B., Schröder, S., Hübers, H.-W. A (2018) Miniaturized Raman/LIBS Instrument for in-Situ Investigation of Celestial Bodies in Pioneering Missions. European Planetary Science Congress, 12: EPSC2018–789.
Mittal, A. K., Chisti, Y., & Banerjee, U. C. (2013). Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnology Advances, 31(2), 346–356. https://doi.org/10.1016/j.biotechadv.2013.01.003
Zannatul, F., & Nemmar, A. (2020). Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. International Journal of Molecular Sciences, 21(7), 2375. https://doi.org/10.3390/ijms21072375
Mashwani, Z. U., Khan, M. A., & Khan, T. (2016). Nadhman, A. Applications of Plant Terpenoids in the Synthesis of Colloidal Silver Nanoparticles. Advances in Colloid and Interface Science, 234, 132–141. https://doi.org/10.1016/j.cis.2016.04.008
Mohamad, N. A. N., Arham, N. A., Jai, J., & Hadi, A. (2014). Plant Extract as Reducing Agent in Synthesis of Metallic Nanoparticles: A Review. Advanced Materials Research, 832, 350–355. https://doi.org/10.4028/www.scientific.net/AMR.832.350
Saeb, A. T. M., Alshammari, A. S., Al-Brahim, H., & Al-Rubeaan, K. A. (2014). Production of Silver Nanoparticles with Strong and Stable Antimicrobial Activity against Highly Pathogenic and Multidrug Resistant Bacteria. ScientificWorldJournal, 2014, 704708. https://doi.org/10.1155/2014/704708
Cameron, S. J., Hosseinian, F., & Willmore, W. G. (2018). A Current Overview of the Biological and Cellular Effects of Nanosilver. International Journal of Molecular Sciences, 19, 2030. https://doi.org/10.3390/ijms19072030
Shawkey, A. M., Rabeh, M. A., Abdulall, A. K., & Abdellatif, A. O. (2013). Green Nanotechnology: Anticancer Activity of Silver Nanoparticles Using Citrullus Colocynthis Aqueous Extracts. Advances in Life Science and Technology, 13, 60–70.
Kathiravan, V., Ravi, S., & Ashokkumar, S. (2014). Synthesis of Silver Nanoparticles from Melia Dubia Leaf Extract and Their in Vitro Anticancer Activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 130, 116–121. https://doi.org/10.1016/J.SAA.2014.03.107
Reddy, N. J., Nagoor Vali, D., Rani, M., & Rani, S. S. (2014). Evaluation of Antioxidant, Antibacterial and Cytotoxic Effects of Green Synthesized Silver Nanoparticles by Piper Longum Fruit. Materials Science and Engineering: C, 34(1), 115–122. https://doi.org/10.1016/J.MSEC.2013.08.039
Heydari, R., & Rashidipour, M. (2015). Green Synthesis of Silver Nanoparticles Using Extract of Oak Fruit Hull (Jaft) Synthesis and in Vitro Cytotoxic Effect on MCF-7 Cells. International Journal of Breast Cancer, 2015(Article ID 846743), 6. https://doi.org/10.1155/2015/846743
Ramar, M., Manikandan, B., Marimuthu, P. N., Raman, T., Mahalingam, A., Subramanian, P., Karthick, S., & Munusamy, A. (2015). Synthesis of Silver Nanoparticles Using Solanum Trilobatum Fruits Extract and Its Antibacterial, Cytotoxic Activity against Human Breast Cancer Cell Line MCF 7. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 140, 223–228. https://doi.org/10.1016/J.SAA.2014.12.060
Arunachalam, K. D., Arun, L. B., Annamalai, S. K., & Arunachalam, A. M. (2015). Potential Anticancer Properties of Bioactive Compounds of Gymnema Sylvestre and Its Biofunctionalized Silver Nanoparticles. International Journal of Nanomedicine, 10, 31. https://doi.org/10.2147/IJN.S71182
Manikandan, R., Manikandan, B., Raman, T., Arunagirinathan, K., Prabhu, N. M., Jothi Basu, M., Perumal, M., Palanisamy, S., & Munusamy, A. (2015). Biosynthesis of Silver Nanoparticles Using Ethanolic Petals Extract of Rosa Indica and Characterization of Its Antibacterial, Anticancer and Anti-Inflammatory Activities. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 138, 120–129. https://doi.org/10.1016/J.SAA.2014.10.043
Palaniappan, P., Sathishkumar, G., & Sankar, R. (2015). Fabrication of Nano-Silver Particles Using Cymodocea Serrulata and Its Cytotoxicity Effect against Human Lung Cancer A549 Cells Line. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 138, 885–890. https://doi.org/10.1016/J.SAA.2014.10.072
Venkatesan, B., Subramanian, V., Tumala, A., & Vellaichamy, E. (2014). Rapid Synthesis of Biocompatible Silver Nanoparticles Using Aqueous Extract of Rosa Damascena Petals and Evaluation of Their Anticancer Activity. Asian Pacific Journal of Tropical Medicine, 7(S1), S294–S300. https://doi.org/10.1016/S1995-7645(14)60249-2
Venugopal, K., Ahmad, H., Manikandan, E., Thanigai Arul, K., Kavitha, K., Moodley, M. K., Rajagopal, K., Balabhaskar, R., & Bhaskar, M. (2017). The Impact of Anticancer Activity upon Beta Vulgaris Extract Mediated Biosynthesized Silver Nanoparticles (Ag-NPs) against Human Breast (MCF-7), Lung (A549) and Pharynx (Hep-2) Cancer Cell Lines. Journal of Photochemistry and Photobiology B: Biology, 173(February), 99–107. https://doi.org/10.1016/j.jphotobiol.2017.05.031
Zhang, D., Ramachandran, G., Mothana, R. A., Siddiqui, N. A., Ullah, R., Almarfadi, O. M., Rajivgandhi, G., & Manoharan, N. (2020). Biosynthesized Silver Nanoparticles Using Caulerpa Taxifolia against A549 Lung Cancer Cell Line through Cytotoxicity Effect/Morphological Damage. Saudi Journal of Biological Sciences, 27(12), 3421–3427. https://doi.org/10.1016/j.sjbs.2020.09.017
Chanthini, A. B., Balasubramani, G., Ramkumar, R., Sowmiya, R., Balakumaran, M. D., Kalaichelvan, P. T., & Perumal, P. (2015). Structural Characterization, Antioxidant and in Vitro Cytotoxic Properties of Seagrass, Cymodocea Serrulata (R.Br.) Asch. & Magnus Mediated Silver Nanoparticles. Journal of Photochemistry and Photobiology B: Biology, 153, 145–152. https://doi.org/10.1016/J.JPHOTOBIOL.2015.09.014
Sukirtha, R., Manasa Priyanka, K., Antony, J. J., Kamalakkannan, S., Thangam, R., Gunasekaran, P., Krishnan, M., & Achiraman, S. (2011). Cytotoxic Effect of Green Synthesized Silver Nanoparticles Using Melia Azedarach against in Vitro HeLa Cell Lines and Lymphoma Mice Model. Process Biochemistry, 47, 273–279. https://doi.org/10.1016/j.procbio.2011.11.003
Kumar, B., Smita, K., Seqqat, R., Benalcazar, K., Grijalva, M., & Cumbal, L. (2016). In Vitro Evaluation of Silver Nanoparticles Cytotoxicity on Hepatic Cancer (Hep-G2) Cell Line and Their Antioxidant Activity: Green Approach for Fabrication and Application. Journal of Photochemistry and Photobiology B: Biology, 159, 8–13. https://doi.org/10.1016/J.JPHOTOBIOL.2016.03.011
Balkrishna, A., Sharma, V. K., Das, S. K., Mishra, N., Bisht, L., Joshi, A., & Sharma, N. (2020). Characterization and Anti-Cancerous Effect of Putranjiva Roxburghii Seed Extract Mediated Silver Nanoparticles on Human Colon (HCT-116), Pancreatic (PANC-1) and Breast (MDA-MB 231) Cancer Cell Lines: A Comparative Study. International Journal of Nanomedicine, 15, 573–585. https://doi.org/10.2147/IJN.S230244
Devi, J. S., Bhimba, V., & Ratnam, K. (2012). Anticancer activity of silver nanoparticles synthesized by the seaweed Ulva lactuca in vitro. Science and Reports, 1, 242–248.
Khanra, K., Panja, S., Choudhuri, I., Chakraborty, A., & Bhattacharyya, N. (2016). Antimicrobial and Cytotoxicity Effect of Silver Nanoparticle Synthesized by Croton Bonplandianum Baill. Leaves Nanomed Journal, 3(1), 15–22. https://doi.org/10.7508/nmj.2016.01.002
Khanra, K., Panja, S., Choudhuri, I., Chakraborty, A., & Bhattacharyya, N. (2015). Evaluation of Antibacterial Activity and Cytotoxicity of Green Synthesized Silver Nanoparticles Using Scoparia Dulcis. Nano Biomedicine and Engineering, 7(3), 128–133. https://doi.org/10.5101/NBE.V7I3.P128-133
Nayak, D., Pradhan, S., Ashe, S., Rauta, P. R., & Nayak, B. (2015). Biologically Synthesised Silver Nanoparticles from Three Diverse Family of Plant Extracts and Their Anticancer Activity against Epidermoid A431 Carcinoma. Journal of Colloid and Interface Science, 457, 329–338. https://doi.org/10.1016/J.JCIS.2015.07.012
Bhattacharya, A., Li, Y., Wade, K. L., Paonessa, J. D., Fahey, J. W., & Zhang, Y. (2010). Allyl Isothiocyanate-Rich Mustard Seed Powder Inhibits Bladder Cancer Growth and Muscle Invasion. Carcinogenesis, 31(12), 2105–2110. https://doi.org/10.1093/carcin/bgq202
Lau, W. S., Chen, T., & Wong, Y. S. (2010). Allyl Isothiocyanate Induces G2/M Arrest in Human Colorectal Adenocarcinoma SW620 Cells through down-Regulation of Cdc25B and Cdc25C. Molecular Medicine Reports, 3(6), 1023–1030. https://doi.org/10.3892/mmr.2010.363
Ching-Lung, L., Shu-Fen, P., Jaw-Chyun, C., Po-Yuan, C., An-Cheng, H., Jin-Cherng, L., Fu-Shin, C., Tai-An, C., Ping-Ping, W., & Kun-IL. (2021). Allyl Isothiocyanate Induces DNA Damage and Impairs DNA Repair in Human Breast Cancer MCF-7 Cells. Anticancer Research, 41(9), 4343–4351.
Muhammad, N., Zhao, H., Song, W., Gu, M., Li, Q., Liu, Y., Li, C., Wang, J., & Zhan, H. (2020). Silver Nanoparticles Functionalized Paclitaxel Nanocrystals Enhance Overall Anti-Cancer Effect on Human Cancer Cells. Nanotechnology, 32(8), 085105. https://doi.org/10.1088/1361-6528/ABCACB
Yuan, Y. G., Zhang, S., Hwang, J. Y., & Kong, I. K. (2018). Silver Nanoparticles Potentiates Cytotoxicity and Apoptotic Potential of Camptothecin in Human Cervical Cancer Cells. Oxidative Medicine and Cellular Longevity, 2018, 6121328. https://doi.org/10.1155/2018/6121328
Semira, S., Daniel, E., & Armand, K. (2021). Prodrugs and prodrug-activated systems in gene therapy. Molecular Therapy, 29(5), 1716–1728. https://doi.org/10.1016/j.ymthe.2021.04.006
Funding
This work is partially supported by the National Institute of Food and Agriculture—AFRI project 1022369 and Hatch project 1019802.
Author information
Authors and Affiliations
Contributions
Conceptualization: C.-A.P.; Methodology and Investigation: A.T., C.-A.P.; Formal analysis and Validation: A.T.; Visualization: A.T.; Supervision: C.-A.P.; Writing – original draft: A.T.; Writing – review and editing: A.T., C.-A.P.
Corresponding author
Ethics declarations
Competing Interests
Authors declare that they have no conflict of interest.
Ethics Approval
No study included human or animal subjects. The study involved the use of A549 lung cancer cells and plasmids was reviewed and approved by the University of Idaho Biosafety Committee with IBC-22–028 protocol.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tarar, A., Peng, CA. Antineoplastic Potency of Sinigrin-functionalized Silver Nanoparticles on Tumor Cells Transfected with Myrosinase-encoded Plasmids. BioNanoSci. 14, 81–92 (2024). https://doi.org/10.1007/s12668-023-01233-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01233-8