Abstract
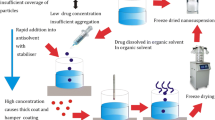
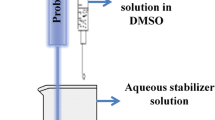
Cefuroxime axetil is a significantly used in drug formulation. Therefore, this study aimed to prepare a nanosuspension of cefuroxime axetil, a poorly water-soluble drug, using an antisolvent precipitation method, followed by ultrasonication. A 32 factorial design was used, and the effects of stirring speed (X1) and poloxamer 188 concentration (X2) on the particle size (Y1) and entrapment efficiency (Y2; %EE) of the prepared nanosuspension were investigated. The nanoparticles were characterized using scanning electron microscopy (SEM), Fourier transform infrared spectrophotometry (FTIR), powder X-ray diffraction (XRD), differential scanning calorimetry (DSC), and zeta potential analysis. The bioavailability of the formulation was assessed in rats. The results indicated that increasing the stirring speed and reducing the poloxamer concentration decreased the size of the nanoparticles. The optimized formulation showed particle size and zeta potential of 170 nm and − 31.3 mV, respectively. FTIR analysis revealed no incompatibilities between the formulation components. XRD and DSC analyses confirmed the amorphization of cefuroxime axetil in the nanosuspension. The developed nanoformulation showed 10.98-fold improvement in the oral bioavailability of cefuroxime axetil. The anti-solvent precipitation process successfully produced a stable amorphous nanosuspension with a notably reduced particle size and improved bioavailability by evaluating and optimizing the critical process and formulation parameters.








Similar content being viewed by others
Data Availability
The data and material will be available on reasonable request.
References
Dhumal, R. S., Biradar, S. V., Yamamura, S., Paradkar, A. R., & York, P. (2008). Preparation of amorphous cefuroxime axetil nanoparticles by sonoprecipitation for enhancement of bioavailability. European Journal of Pharmaceutics and Biopharmaceutics 70, 109–115. https://doi.org/10.1016/j.ejpb.2008.04.001
Rouge, N., Buri, P., & Doelker, E. (1996). Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. International Journal of Pharmaceutics, 136, 117–139. https://doi.org/10.1016/0378-5173(96)85200-8
Finn, A., Straughn, A., Meyer, M., & Chubb, J. (1987). Effect of dose and food on the bioavailability of cefuroxime axetil. Biopharmaceutics & Drug Disposition, 8(6), 519–526. https://doi.org/10.1002/bdd.2510080604
Khan, I., Saeed, K., & Khan, I. (2019). Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry, 12(7), 908–931. https://doi.org/10.1016/j.arabjc.2017.05.011
Nadaf, S. J., Killedar, S. G., Kumbar, V. M., Bhagwat, D. A., & Gurav, S. S. (2022). Pazopanib-laden lipid based nanovesicular delivery with augmented oral bioavailability and therapeutic efficacy against non-small cell lung cancer. International Journal of Pharmaceutics, 628, 122287. https://doi.org/10.1016/j.ijpharm.2022.122287
Kumbhar, P., Waghmare, P., Nadaf, S. J., Manjappa, A., Shah, R., & Disouza, J. (2023). QbD and Six Sigma quality approach for chromatographic estimation of repurposed simvastatin from nanostructured lipid carriers. Microchemical Journal, 185, 108310. https://doi.org/10.1016/j.microc.2022.108310
Patel, D., Zode, S. S., & Bansal, A. K. (2020). Formulation aspects of intravenous nanosuspensions. International Journal of Pharmaceutics, 586, 119555. https://doi.org/10.1016/j.ijpharm.2020.119555
Patravale, V. B., Date, A. A., & Kulkarni, R. M. (2004). Nanosuspensions: A promising drug delivery strategy. Journal of Pharmacy and Pharmacology, 56(7), 827–840. https://doi.org/10.1211/0022357023691
Yadav, S. K., Mishra, S., & Mishra, B. (2012). Eudragit-based nanosuspension of poorly water-soluble drug: Formulation and in vitro-in vivo evaluation. An Official Journal of the American Association of Pharmaceutical Scientists, 13(4), 1031–1044. https://doi.org/10.1208/s12249-012-9833-0
Qiao, H., Chen, L., Rui, T., Wang, J., Chen, T., Fu, T., Li, J., & Di, L. (2017). Fabrication and in vitro/in vivo evaluation of amorphous andrographolide nanosuspensions stabilized by d-α-tocopheryl polyethylene glycol 1000 succinate/sodium lauryl sulfate. International Journal of Nanomedicine, 12, 1033–1046. https://doi.org/10.2147/IJN.S120887
Hong, C., Dang, Y., Lin, G., Yao, Y., Li, G., Shen, H., & Xie, Y. (2014). Effects of stabilizing agents on the development of myricetin nanosuspension and its characterization: An in vitro and in vivo evaluation. International Journal of Pharmaceutics, 477(1–2), 251–260. https://doi.org/10.1016/j.ijpharm.2014.10.044
Luo, Y., Xu, L., Tao, X., Xu, M., Feng, J., & Tang, X. (2013). Preparation, characterization, stability and in vitro-in vivo evaluation of pellet-layered simvastatin nanosuspensions. Drug Development and Industrial Pharmacy, 39(7), 936–946. https://doi.org/10.3109/03639045.2012.699067
Nadaf, S. J., & Killedar, S. G. (2018). Curcumin nanocochleates: Use of design of experiments, solid state characterization, in vitro apoptosis and cytotoxicity against breast cancer MCF-7 cells. Journal of Drug Delivery Science and Technology, 47, 337–350. https://doi.org/10.1016/j.jddst.2018.06.026
Clementino, A., & Sonvico, F. (2018). Development and validation of a RP-HPLC method for the simultaneous detection and quantification of simvastatin’s isoforms and coenzyme Q10 in lecithin/chitosan nanoparticles. Journal of Pharmaceutical and Biomedical Analysis, 155, 33–41. https://doi.org/10.1016/j.jpba.2018.03.046
Sun, M., Gao, Y., Pei, Y., Guo, C., Li, H., Cao, F., Yu, A., & Zhai, G. (2010). Development of nanosuspension formulation for oral delivery of quercetin. Journal of Biomedical Nanotechnology, 6(4), 325–332. https://doi.org/10.1166/jbn.2010.1133
Yasmin Begum, M., Saisree, R., Harshitha, P., Shwetha, A., & Sudhakar, M. (2017). Preparation and evaluation of nanosuspension of nifedipine. International Journal of Current Research., 9, 57091–57098.
Talekar, M., Ganta, S., Amiji, M., Jamieson, S., Kendall, J., Denny, W. A., & Garg, S. (2013). Development of PIK-75 nanosuspension formulation with enhanced delivery efficiency and cytotoxicity for targeted anti-cancer therapy. International Journal of Pharmaceutics, 450(1–2), 278–289. https://doi.org/10.1016/j.ijpharm.2013.04.057
Sahu, B. P., & Das, M. K. (2014). Nanosuspension for enhancement of oral bioavailability of felodipine. Applied Nanoscience, 4, 189–197. https://doi.org/10.1007/s13204-012-0188-3
Dessai, S., Ayyanar, M., Amalraj, S., Khanal, P., Vijayakumar, S., Gurav, N., Rarokar, N., Kalaskar, M., Nadaf, S., & Gurav, S. (2022). Bioflavonoid mediated synthesis of TiO2 nanoparticles: Characterization and their biomedical applications. Materials Letters, 311, 131639. https://doi.org/10.1016/j.matlet.2021.131639
Dias, C., Ayyanar, M. S., Amalraj, S., Khanal, P., Subramaniyan, V., Das, S., Gandhale, P., Biswa, V., Ali, R., Gurav, N., Nadaf, S., Rarokar, N., & Gurav, S. (2022). Biogenic synthesis of zinc oxide nanoparticles using mushroom fungus Cordyceps militaris: characterization and mechanistic insights of therapeutic investigation. Journal of Drug Delivery Science and Technology, 73, 103444. https://doi.org/10.1016/j.jddst.2022.103444
Killedar, S. G., Nale, A. B., More, H. N., Nadaf, S. J., Pawar, A. A., & Tamboli, U. S. (2014). Isolation, characterization, and evaluation of Cassia fistula Linn. seed and pulp polymer for pharmaceutical application. International Journal of Pharmaceutical Investigation, 4(4), 215–225. https://doi.org/10.4103/2230-973X.143128
Nadaf, S., & Killedar, S. (2020). Development and validation of RP-HPLC method for estimation of curcumin from nanocochleates and its application in in-vivo pharmacokinetic study. Acta Chimica Slovenica, 67(4), 1100–1110.
Na, Y. G., Pham, T. M. A., Byeon, J. J., Kim, M. K., Han, M. G., Baek, J. S., Lee, H. K., & Cho, C. W. (2020). Development and evaluation of TPGS/PVA-based nanosuspension for enhancing dissolution and oral bioavailability of ticagrelor. International Journal of Pharmaceutics, 581, 119287. https://doi.org/10.1016/j.ijpharm.2020.119287
Massart, D. L., Vandeginste, B. G. M., Deming S. N., Michotte ,Y., Kaufman, L. (Eds) (2003). Exploration of response surfaces. In: Data Handling in Science and Technology. Elsevier, 271–291. https://doi.org/10.1016/S0922-3487(08)70230-2
Jadhav, N. R., Nadaf, S. J., Lohar, D. A., Ghagare, P. S., & Powar, T. A. (2017). Phytochemicals formulated as nanoparticles: Inventions, recent patents and future prospects. Recent Patents on Drug Delivery & Formulation, 11, 173–186. https://doi.org/10.2174/1872211311666171120102531
Yeole, B. D., Patil, R. P., Lone, K. D., & Tekade, A. R. (2016). Preparation of nanoparticles of poorly water-soluble dronedarone by antisolvent addition technique using the natural polymer as a stabilizer. Journal of Pharmaceutical Research and Clinical Practice, 6, 8–16.
Dawood, N. M., Abdal-Hammid, N. R., & Hussien, A. A. (2018). Formulation and characterization of lafutidine nanosuspension for oral drug delivery system. International Journal of Applied Pharmaceutics, 10(2), 20–30.
Sanjula, B., Shah, F. M., Javed, A., & Alka, A. (2009). Effect of poloxamer 188 on lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. Journal of Drug Targeting, 17(3), 249–256. https://doi.org/10.1080/10611860902718672
Agarwal, V., & Bajpai, M. (2014). Preparation and optimization of esomeprazole nanosuspension using evaporative precipitation– ultrasonication. Tropical Journal of Pharmaceutical Research, 13, 497–503.
Zhang, J. Y., Shen, Z. G., Zhong, J., Hu, T. T., Chen, J. F., Ma, Z. Q., & Yun, J. (2006). Preparation of amorphous cefuroxime axetil nanoparticles by controlled nanoprecipitation method without surfactants. International Journal of Pharmaceutics, 323(1–2), 153–160. https://doi.org/10.1016/j.ijpharm.2006.05.048
Gajera, B. Y., Shah, D. A., & Dave, R. H. (2019). Development of an amorphous nanosuspension by sonoprecipitation-formulation and process optimization using design of experiment methodology. International Journal of Pharmaceutics, 559, 348–359. https://doi.org/10.1016/j.ijpharm.2019.01.054
Bhattacharjee, S. (2016). DLS and zeta potential - what they are and what they are not? Journal of Controlled Release, 235, 337–351. https://doi.org/10.1016/j.jconrel.2016.06.017
Patil, A. S., Hegde, R., Gadad, A. P., Dandagi, P. M., Masareddy, R., & Bolmal, U. (2021). Exploring the solvent-anti-solvent method of nanosuspension for enhanced oral bioavailability of lovastatin. Turkish Journal Of Pharmaceutical Sciences, 18(5), 541–549. https://doi.org/10.4274/tjps.galenos.2020.65047
Alhamhoom, Y., Honmane, S. M., Hani, U., Osmani, R. A. M., Kandasamy, G., Vasudevan, R., Paramshetti, S., Dudhal, R. R., Kengar, N. K., & Charde, M. S. (2023). Study of formulation and process variables for optimization of piroxicam nanosuspension using 32 factorial design to improve solubility and in vitro bioavailability. Polymers, 15(3), 483. https://doi.org/10.3390/polym15030483
Kolipaka, E., Sen, S., Mane, S. S., Bajad, G. D., Dengale, S. J., Ranjan, O. P. (2023). Development of posaconazole nanosuspension for bioavailability enhancement: formulation optimization, in vitro characterization, and pharmacokinetic study. Journal of Drug Delivery Science and Technology, 83:104434, ISSN 1773–2247. https://doi.org/10.1016/j.jddst.2023.104434
Jadhav, N. R., Tone, J. S., Irny, P. V., & Nadaf, S. J. (2013). Development and characterization of gelatin based nanoparticles for targeted delivery of zidovudine. Int J Pharm Investig., 3(3), 126–130. https://doi.org/10.4103/2230-973X.119213
Kousar, F., Jahan, N., Sultana, B., Khalil-Ur-Rahman (2022). Optimization of process parameters by response surface methodology to develop a more bioefficacious nanosuspension of Silybum marianum seed extract. Tropical Journal of Pharmaceutical Research, 21(7):1365–1375. https://doi.org/10.4314/tjpr.v21i7.2
Hussain, A., Attique, F., Naqvi, S. A. R., Ali, A., Ibrahim, M., Hussain, H., Zafar, F., Iqbal, R. S., Ayub, M. A., Assiri, M. A., Imran, M., & Ullah, S. (2022). Nanoformulation of Curcuma longa root extract and evaluation of ıts dissolution potential. ACS Omega, 8(1), 1088–1096. https://doi.org/10.1021/acsomega.2c06258
Sankari, T., & Al-Hariri, S. (2018). Preparation and characterization of cefuroxime axetil solid dispersions using poloxamer 188. Brazilian Journal of Pharmaceutical Sciences, 54, 17644.
Marimuthu, S., Nantheeswaran, S., Shanmugarathinam, A., & Purachikody, A. (2012). Formulation and characterization of cefuroxime axetil floating microspheres. International Journal of Pharmaceutics, 2(3), 526–533.
Acknowledgements
This work is a Ph.D. research work of Mr. Haragouri Mishra registered under Centurion University of Technology and Management, Odisha, under the guidance of Dr. Amulyaratna Behra and Dr. Sidharth S. Kar.
Funding
None.
Author information
Authors and Affiliations
Contributions
Concept — H.M., S.K.; design — H.M., S.D., S.K.; supervision — A.B.; resources —A.B., S.D.; materials — A.B.; data collection and/or processing — H.M., A.B.; analysis and/or interpretation — H.M., A.B., S.K., S.D.; literature search — H.M. A.B., S.D.; writing — H.M.; critical reviews — H.M., A.B., S.K., S.D, S.M and SS.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Research Involving Humans and Animals Statement
Authors have followed all applicable international, national, and/or institutional guidelines for the care and use of animals. The animal studies were accomplished according to CPCSEA guidelines. The animal studies were approved by the Centurion University of Technology and Management, Odisha, India.
Informed Consent
We, all the author declares that there are no conflicts of interest among us. All the research facilities were provided by Centurion University of Technology and Management, Odisha. The animal experimentation was conducted as per the guidelines of CPCSEA (2024/PO/Re/S/18/CPCSEA) and approved by IAEC (CUTM/IAEC-03 dated 27/03/2023) of CUTM.
Consent for Publication
Not applicable.
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, H., Behera, A., Kar, S.S. et al. Development and Optimization of Cefuroxime Axetil Nanosuspension for Improved Oral Bioavailability: In-Vitro and In-Vivo Investigations. BioNanoSci. 13, 2371–2384 (2023). https://doi.org/10.1007/s12668-023-01214-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01214-x