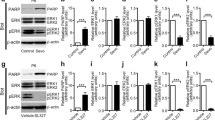
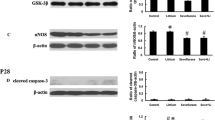
Abstract
Sevoflurane (Sev) is a widely used inhalational anesthetic for general anesthesia in children. Previous studies have confirmed that multiple exposures to inhaled anesthetic can induce long-term neurotoxicity in newborn mice. However, the underlying mechanisms remain elusive. In this study, we investigated the role of homeodomain interacting protein kinase 2 (HIPK2), a stress activating kinase involved in neural survival and synaptic plasticity, and its underlying mechanism in sevoflurane-induced neurotoxicity. Empirical study showed that neuronal apoptosis was elevated after exposure to sevoflurane. Meanwhile, up-regulation of HIPK2 and AKT/mTOR signaling was observed in primary hippocampal neurons and hippocampus in mice upon anesthetic exposure. A64, antagonist of HIPK2, could significantly reduce increased apoptosis and activation of AKT/mTOR induced by sevoflurane. AKT antagonist MK2206 partially alleviated neuronal apoptosis without affecting the expression of HIPK2. Experimental results demonstrated a crucial role of HIPK2/AKT/mTOR signaling in neurotoxicity of sevoflurane. Thus, HIPK2/AKT/mTOR signaling can serve as a potential target for the protection of inhalation anesthesia-induced cytotoxicity in the future.






Similar content being viewed by others
References
Bahmad H, Darwish B, Dargham K, Machmouchi R, Dargham B, Osman M, Khechen Z, El Housheimi N, Abou-Kheir W, Chamaa F (2020) Role of MicroRNAs in anesthesia-induced neurotoxicity in animal models and neuronal cultures: a systematic review. Neurotox Res 37(3):479–90. https://doi.org/10.1007/s12640-019-00135-6
Bon G, Di Carlo S, Folgiero V, Avetrani P, Lazzari C, D'Orazi G, Brizzi M, Sacchi A, Soddu S, Blandino G, Mottolese M, Falcioni R (2009) Negative regulation of beta4 integrin transcription by homeodomain-interacting protein kinase 2 and p53 impairs tumor progression. Cancer Res 69(14):5978–86. https://doi.org/10.1158/0008-5472.can-09-0244
Chalazonitis A, Tang A, Shang Y, Pham T, Hsieh I, Setlik W, Gershon M, Huang E (2011) Homeodomain interacting protein kinase 2 regulates postnatal development of enteric dopaminergic neurons and glia via BMP signaling. J Neurosci Off J Soc Neurosci 31(39):13746–57. https://doi.org/10.1523/jneurosci.1078-11.2011
Chen Y, Nie H, Tian L, Tong L, Deng J, Zhang Y, Dong H, Xiong L (2015) Sevoflurane preconditioning-induced neuroprotection is associated with Akt activation via carboxy-terminal modulator protein inhibition. Br J Anaesth 114(2):327–35. https://doi.org/10.1093/bja/aeu271
Dong Y, Hong W, Tang Z, Gao Y, Wu X, Liu H (2020) Dexmedetomidine attenuates neurotoxicity in developing rats induced by sevoflurane through upregulating BDNF-TrkB-CREB and downregulating ProBDNF-P75NRT-RhoA signaling pathway. Mediat Inflamm 2020:5458061. https://doi.org/10.1155/2020/5458061
Glatz P, Sandin R, Pedersen N, Bonamy A, Eriksson L, Granath F (2017) Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr 171(1):e163470. https://doi.org/10.1001/jamapediatrics.2016.3470
Guo M, Zhu X, Xu H, Li J, Yang S, Zuo Z, Lin D (2019) Ulinastatin attenuates isoflurane-induced cognitive dysfunction in aged rats by inhibiting neuroinflammation and β-amyloid peptide expression in the brain. Neurol Res 41(10):923–9. https://doi.org/10.1080/01616412.2019.1642564
Ing C, Jackson W, Zaccariello M, Goldberg T, McCann M, Grobler A, Davidson A, Sun L, Li G, Warner D (2020) Prospectively assessed neurodevelopmental outcomes in studies of anaesthetic neurotoxicity in children: a systematic review and meta-analysis. Br J Anaesth. https://doi.org/10.1016/j.bja.2020.10.022
Lee S, Shang Y, Redmond S, Urisman A, Tang A, Li K, Burlingame A, Pak R, Jovičić A, Gitler A, Wang J, Gray N, Seeley W, Siddique T, Bigio E, Lee V, Trojanowski J, Chan J, Huang E (2016) Activation of HIPK2 promotes ER stress-mediated neurodegeneration in amyotrophic lateral sclerosis. Neuron 91(1):41–55. https://doi.org/10.1016/j.neuron.2016.05.021
Liang L, Xie R, Lu R, Ma R, Wang X, Wang F, Liu B, Wu S, Wang Y, Zhang H (2020) Involvement of homodomain interacting protein kinase 2-c-Jun N-terminal kinase/c-Jun cascade in the long-term synaptic toxicity and cognition impairment induced by neonatal Sevoflurane exposure. J Neurochem 154(4):372–88. https://doi.org/10.1111/jnc.14910
Liu B, Bai W, Ou G, Zhang J (2019) Cdh1-mediated metabolic switch from pentose phosphate pathway to glycolysis contributes to sevoflurane-induced neuronal apoptosis in developing brain. ACS Chem Neurosci 10(5):2332–44. https://doi.org/10.1021/acschemneuro.8b00644
Liu F, Liu C, Xu M, Zhu J, Yu W, Wang L (2020) Effects of sevoflurane postconditioning on the expression of AKT/mTOR and apoptosis of myocardial cells in myocardial ischemia-reperfusion rats. J Biol Regul Homeost Agents 34(5):1909–13. https://doi.org/10.23812/20-429-l
Li Y, Lv Y, Cheng C, Huang Y, Yang L, He J, Tao X, Hu Y, Ma Y, Su Y, Wu L, Yu G, Jiang Q, Liu S, Liu X, Liu Z (2020) SPEN induces miR-4652–3p to target HIPK2 in nasopharyngeal carcinoma. Cell Death Dis 11(7):509. https://doi.org/10.1038/s41419-020-2699-2
McCann M, Soriano S (2019) Does general anesthesia affect neurodevelopment in infants and children? BMJ (Clinical Research ed). 367:l6459. https://doi.org/10.1136/bmj.l6459
Milanovic D, Pesic V, Popic J, Tanic N, Kanazir S, Jevtovic-Todorovic V, Ruzdijic S (2014) Propofol anesthesia induces proapoptotic tumor necrosis factor-alpha and pro-nerve growth factor signaling and prosurvival Akt and XIAP expression in neonatal rat brain. J Neurosci Res. 92(10):1362–73. https://doi.org/10.1002/jnr.23409
Neag M, Mitre A, Catinean A, Mitre C (2020) An overview on the mechanisms of neuroprotection and neurotoxicity of isoflurane and sevoflurane in experimental studies. Brain Res Bull 165:281–9. https://doi.org/10.1016/j.brainresbull.2020.10.011
Nkpaa K, Owoeye O, Amadi B, Adedara I, Abolaji A, Wegwu M, Farombi E (2020) Ethanol exacerbates manganese-induced oxidative/nitrosative stress, pro-inflammatory cytokines, nuclear factor-κB activation, and apoptosis induction in rat cerebellar cortex. J Biochem Mol Toxicol e22681. https://doi.org/10.1002/jbt.22681
Pollard R, Hopkins T, Smith C, May B, Doyle J, Chambers C, Clark R, Buhrman W (2018) Perianesthetic and anesthesia-related mortality in a southeastern United States population: a longitudinal review of a prospectively collected quality assurance data base. Anesth Analg 127(3):730–5. https://doi.org/10.1213/ane.0000000000003483
Rosenholm M, Paro E, Antila H, Voikar V, Rantamaki T (2017) Repeated brief isoflurane anesthesia during early postnatal development produces negligible changes on adult behavior in male mice. PLoS One 12(4):e0175258. https://doi.org/10.1371/journal.pone.0175258
Saul V, Schmitz M (2013) Posttranslational modifications regulate HIPK2, a driver of proliferative diseases. J Mol Med (Berlin, Germany). 91(9):1051–8. https://doi.org/10.1007/s00109-013-1042-0
Shan Y, Sun S, Yang F, Shang N, Liu H (2018) Dexmedetomidine protects the developing rat brain against the neurotoxicity wrought by sevoflurane: role of autophagy and Drp1-Bax signaling. Drug Des Devel Ther 12:3617–24. https://doi.org/10.2147/dddt.s180343
Shang Y, Zhang J, Huang E (2018) HIPK2-mediated transcriptional control of NMDA receptor subunit expression regulates neuronal survival and cell death. J Neurosci Off J Soc Neurosci 38(16):4006–19. https://doi.org/10.1523/jneurosci.3577-17.2018
Shi C, Jin J, Wang X, Song T, Li G, Li K, Ma J (2020) Sevoflurane attenuates brain damage through inhibiting autophagy and apoptosis in cerebral ischemia‑reperfusion rats. Mol Med Rep 21(1):123–30. https://doi.org/10.3892/mmr.2019.10832
Shi Y, Hu D, Rodgers E, Katusic S, Gleich S, Hanson A, Schroeder D, Flick R, Warner D (2018) Epidemiology of general anesthesia prior to age 3 in a population-based birth cohort. Paediatr Anaesth 28(6):513–9. https://doi.org/10.1111/pan.13359
Stefani L, Gamermann P, Backof A, Guollo F, Borges R, Martin A, Caumo W, Felix E (2018) Perioperative mortality related to anesthesia within 48 h and up to 30 days following surgery: A retrospective cohort study of 11,562 anesthetic procedures. J Clin Anesth 49:79–86. https://doi.org/10.1016/j.jclinane.2018.06.025
Sun L (2010) Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth i61–8. https://doi.org/10.1093/bja/aeq302
Vinson A, Houck C (2018) Neurotoxicity of anesthesia in children: prevention and treatment. Curr Treat Options Neurol 20(12):51. https://doi.org/10.1007/s11940-018-0536-z
Vutskits L, Xie Z (2016) Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci 17(11):705–17. https://doi.org/10.1038/nrn.2016.128
Wang N, Wang M (2019) Dexmedetomidine suppresses sevoflurane anesthesia-induced neuroinflammation through activation of the PI3K/Akt/mTOR pathway. BMC Anesthesiol 19(1):134. https://doi.org/10.1186/s12871-019-0808-5
Xu Z, Qian B (2020) Sevoflurane anesthesia-mediated oxidative stress and cognitive impairment in hippocampal neurons of old rats can be ameliorated by expression of brain derived neurotrophic factor. Neurosci Lett 721:134785. https://doi.org/10.1016/j.neulet.2020.134785
Ye Z, Xia P, Cheng Z, Guo Q (2015) Neuroprotection induced by sevoflurane-delayed post-conditioning is attributable to increased phosphorylation of mitochondrial GSK-3β through the PI3K/Akt survival pathway. J Neurol Sci 348:216–25. https://doi.org/10.1016/j.jns.2014.12.011
Zhang J, Dong Y, Zhou C, Zhang Y, Xie Z (2015) Anesthetic sevoflurane reduces levels of hippocalcin and postsynaptic density protein 95. Mol Neurobiol 51(3):853–63. https://doi.org/10.1007/s12035-014-8746-1
Zhou H, Li S, Wang G (2019) Euxanthone ameliorates sevoflurane-induced neurotoxicity in neonatal mice. J Mol Neurosci: MN. 68(2):275–86. https://doi.org/10.1007/s12031-019-01303-1
Zhu X, Yao Y, Guo M, Li J, Yang P, Xu H, Lin D (2020) Sevoflurane increases intracellular calcium to induce mitochondrial injury and neuroapoptosis. Toxicol Lett 336:11–20. https://doi.org/10.1016/j.toxlet.2020.11.002
Acknowledgements
The authors thank Drs. Andi Cheng and Yuanyuan Zhu for their technical assistance.
Funding
This work was supported by National Natural Science Foundation of China to Dr. Hui Zhang (Grant No. 81971076, 81371265), Science Innovation Promoting Program of Shaanxi Province to Dr. Hui Zhang (Grant No. 2014KTCL0305), Natural Science of Shaanxi Province to Ms. Liang (Grant No. 2021JQ345), and National Natural Science Foundation of China to Ms. Liang (Grant No. 82101345).
Author information
Authors and Affiliations
Contributions
Flow cytometry apoptosis detection: L.L., F.Z. H.D.; immunohistochemistry: L.L., Z.Y., M.T.; Western blotting: L.L. K.J., F.Z.; data analysis: L.L., H.D., L.B.; manuscript preparation: L.L., Z.H.; experimental design: L.L., Z.H.; financial support: Z.H., L.L.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethical Approval and Consent to Participate.
All experimental procedures were carried out according to the protocols approved by the Animal Care and Use Committee of FMMU.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, L., Fan, Z., He, D. et al. Sevoflurane-Induced Neurotoxicity in the Developing Hippocampus via HIPK2/AKT/mTOR Signaling. Neurotox Res 40, 803–813 (2022). https://doi.org/10.1007/s12640-021-00445-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00445-8