Abstract
Purpose
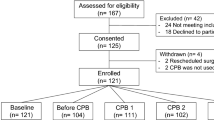
While the Nova StatStrip® Glucose Hospital Meter System (Nova Biomedical, Waltham, MA, USA) is approved for point-of-care testing (POCT) in critically ill patients, its use during major abdominal surgery has not been evaluated. The purpose of this study was to assess the accuracy of the Nova StatStrip glucometer in patients undergoing major hepatobiliary procedures using the Parkes error grid (ISO15197:2013) and criteria defined by the Clinical and Laboratory Standards Institute (CLSI) POCT12-A3 guideline.
Methods
This study was a post hoc exploratory study of patients participating in a prospective randomized controlled trial on the effects of hyperinsulinemic normoglycemia (HNC) on infectious outcomes after hepatobiliary surgery. Arterial blood samples were collected before surgery and one hour, two hours, and three hours after baseline. Blood glucose levels were analyzed by the Nova StatStrip glucometer and the GEM® PremierTM 5000 blood gas analyzer. Accuracy of the StatStrip glucometer was assessed using the Parkes error grid for type 1 diabetes mellitus (when 99% of samples were within zones A and B on the Parkes error grid and clinical accuracy was acceptable) and the CLSI POCT12-A3 criteria.
Results
Blood glucose levels were analyzed in 135 patients, 70 of whom received the HNC. In the Parkes error grid plotted, all samples at all time-points were within zones A and B. The Nova StatStrip glucometer also satisfied CLSI POCT12-A3 criteria at all time-points.
Conclusion
The Nova StatStrip glucometer was accurate in patients undergoing major upper abdominal surgery, independent of the administration of high-dose insulin therapy.
Study registration
ClinicalTrials.gov (NCT01528189); registered 7 February 2012.
Résumé
Objectif
Bien que le système hospitalier de lecture de la glycémie StatStrip® de Nova (Nova Biomedical, Waltham, MA, É.-U.) soit approuvé pour une utilisation au chevet (ou POCT, pour ‘Point of Care Testing’) chez la patientèle en état critique, son utilisation n’a pas été évaluée en chirurgie abdominale majeure. L’objectif de cette étude était d’évaluer la précision du glucomètre StatStrip de Nova chez la patientèle bénéficiant d’interventions hépatobiliaires majeures à l’aide de la grille d’erreur de Parkes (ISO15197:2013) et des critères définis par la directive POCT12-A3 du Clinical and Laboratory Standards Institute (CLSI).
Méthode
Il s’agissait d’une étude exploratoire post-hoc auprès de patient·es participant à une étude randomisée contrôlée prospective sur les effets de la normoglycémie hyperinsulinémique (HNC) sur les issues infectieuses après une chirurgie hépatobiliaire. Des échantillons de sang artériel ont été prélevés avant la chirurgie et une heure, deux heures et trois heures après l’échantillon initial. Les taux de glycémie ont été analysés avec le glucomètre StatStrip de Nova et l’analyseur de gaz sanguin GEM® PremierTM 5000. La précision du glucomètre StatStrip a été évaluée à l’aide de la grille d’erreur de Parkes pour le diabète sucré de type 1 (lorsque 99 % des échantillons se trouvaient dans les zones A et B de la grille d’erreur de Parkes et que la précision clinique était acceptable) et des critères POCT12-A3 du CLSI.
Résultats
La glycémie a été analysée chez 135 personnes, dont 70 ont reçu une normoglycémie hyperinsulinémique. Dans la grille d’erreur de Parkes tracée, tous les échantillons à tous les points temporels se trouvaient dans les zones A et B. Le glucomètre StatStrip de Nova a également satisfait aux critères POCT12-A3 du CLSI à tous les points temporels.
Conclusion
Le glucomètre StatStrip de Nova était précis chez la patientèle bénéficiant d’une chirurgie abdominale supérieure majeure, indépendamment de l’administration d’insulinothérapie à forte dose.
Enregistrement de l’étude
ClinicalTrials.gov (NCT01528189); enregistrée le 7 février 2012.



Similar content being viewed by others
References
Peacock TS. Perioperative hyperglycemia: a literature review. AORN J 2019; 109: 80–6. https://doi.org/10.1002/aorn.12445
Girard M, Schricker T. Perioperative glucose control: living in uncertain times—continuing professional development. Can J Anesth 2011; 58: 312–20. https://doi.org/10.1007/s12630-010-9449-3
Nova Biomoedical. StatStrip glucose hospital meter system receives FDA clearance for capillary testing with critically ill patients. Available from URL: https://novabio.us/press/07.16.18.php (accessed May 2023).
Karon BS, Griesmann L, Scott R, et al. Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol Ther 2008; 10: 111–20. https://doi.org/10.1089/dia.2007.0257
Nakadate Y, Sato H, Roque P, et al. Accuracy of blood glucose measurements using the NOVA StatStrip® glucometer during cardiac surgery: a prospective observational study. Can J Anesth 2019; 66: 943–52. https://doi.org/10.1007/s12630-019-01350-7
Karon BS, Donato LJ, Larsen CM, et al. Accuracy of capillary and arterial whole blood glucose measurements using a glucose meter in patients under general anesthesia in the operating room. Anesthesiology 2017; 127: 466–74. https://doi.org/10.1097/aln.0000000000001708
Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 2000; 23: 1143–8. https://doi.org/10.2337/diacare.23.8.1143
Sacks DB. Point-of-Care Blood Glucose Testing in Acute and Chronic Care Facilities: Approved Guideline, 3rd ed. Pennsylvania: Clinical and Laboratory Standards Institute; 2013.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–9. https://doi.org/10.1016/j.ijsu.2014.07.013
Rice MJ, Pitkin AD, Coursin DB. Review article: glucose measurement in the operating room: more complicated than it seems. Anesth Analg 2010; 110: 1056–65. https://doi.org/10.1213/ane.0b013e3181cc07de
Karon BS, Blanshan CT, Deobald GR, Wockenfus AM. Retrospective evaluation of the accuracy of Roche AccuChek Inform and Nova StatStrip® glucose meters when used on critically ill patients. Diabetes Technol Ther 2014; 16: 828–32. https://doi.org/10.1089/dia.2014.0074
Rabiee A, Magruder JT, Grant C, et al. Accuracy and reliability of the Nova StatStrip® glucose meter for real-time blood glucose determinations during glucose clamp studies. J Diabetes Sci Technol 2010; 4: 1195–201. https://doi.org/10.1177/193229681000400519
Thompson MH, Wilson SH, Toussaint BL, et al. Effect of subcutaneous unfractionated heparin prophylaxis on activated partial thromboplastin time: a retrospective evaluation. J Clin Anesth 2016; 33: 346–50. https://doi.org/10.1016/j.jclinane.2015.11.020
Carvalho G, Lattermann R, Codere-Maruyama T, Schricker T. Glucose and insulin administration while maintaining normoglycemia: the GIN concept. Minerva Anestesiol 2013; 79: 74–82.
Cembrowski G. Flawed analytical method used for reference glucose. Can J Anesth 2020; 67: 158–9. https://doi.org/10.1007/s12630-019-01444-2
Nakadate Y, Sato H, Roque P, et al. In reply: flawed analytical method used for reference glucose. Can J Anesth 2020; 67: 160. https://doi.org/10.1007/s12630-019-01445-1
Kattar M, Xu Q, Cembrowski A, Mei J, Sadrzadeh H, Cembrowski GS. Reduced accuracy of GEM 4000 for measurement of electrolytes, glucose, and hemoglobin in relation to calibration schedule. Am J Clin Pathol 2018; 149: S8. https://doi.org/10.1093/ajcp/aqx115.018
Cembrowski G, Jung J, Mei J, et al. Five-year two-center retrospective comparison of central laboratory glucose to GEM 4000 and ABL 800 blood glucose: demonstrating the (in)adequacy of blood gas glucose. J Diabetes Sci Technol 2020; 14: 535–4. https://doi.org/10.1177/1932296819883260
Author contributions
Keisuke Omiya contributed to the acquisition, analysis, and interpretation of data, and drafting the article. Yosuke Nakadate and Hiroaki Sato contributed to conception and design of the study, and analysis and interpretation of data. Bon-Wook Koo contributed to conception and design of the study, and the acquisition and interpretation of data. Thomas Schricker contributed to conception and design of the study, analysis and interpretation of data, and drafting the article.
Disclosures
All authors declare that they have no financial or nonfinancial interests that may be relevant to the submitted work.
Funding statement
This study was supported by funds provided by the Department of Anesthesia at the Royal Victoria Hospital, McGill University Health Centre and McGill University.
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omiya, K., Nakadate, Y., Sato, H. et al. Accuracy of the Nova StatStrip® glucometer in patients undergoing major abdominal surgery: an observational study. Can J Anesth/J Can Anesth 70, 1970–1977 (2023). https://doi.org/10.1007/s12630-023-02606-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02606-z