Abstract
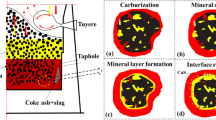
This study investigates the surface of unpolished samples of blast furnace (BF) coke drilled from the tuyere zone, which hosts Fe-Si particles (mostly Fe3Si) that vary in size, shape, depth of submersion (penetration) into the coke matrix, and contact features with the surface. Based on the shape of the particles and the extent of their contact with the coke matrix, they have been grouped into three major types: (I) sphere-like droplets with limited contact area, (II) semi-spheres with a larger contact area, and (III) irregular segregations with a spherical surface, which exhibit the largest contact area among the three types of particles. Considering the ratio between the height (h) of the particles and half of their length at the surface level (l) along the cross-section, these three types can be characterized as follows: (I) h > l, (II) h ≈ l, and (III) h < l. All the three types of particles can be found near each other. The shape and the extent of the contact depend on the degree of penetration of the material into the matrix, which is a function of the composition of the particles. Type (I) particles were initially saturated with Si at an earlier stage and, for that reason, they can react less with carbon in the coke matrix than type (II) and (III), thereby moving faster through the coke cone. Thermodynamic calculations have shown that the temperature interval of 1250–1300°C can be considered the starting point for Si entering into molten iron under quartz-dominated coke ash. Accordingly, the initial pick-up of Si by molten iron can be assumed to be mineral-related. In terms of BF practice, better conditions for sliding Fe-Si droplets through the coke cone are available when they come into contact with free SiO2 concentrated into small grains, and when the SiO2/ΣMe x O y mass ratio in the coke ash is high.
Similar content being viewed by others
References
J. Yagi, Mathematical modeling of the flow of four fluids in a packed bed, ISIJ Int., 33(1993), No. 6, p. 619.
I. Jeong, H. Kim, and Y. Sasaki, Trickle flow behaviors of liquid iron and molten slag in the lower part of blast furnace, ISIJ Int., 53(2013), No. 12, p. 2090.
J. Lacaze and B. Sundman, An assessment of the Fe-C-Si system, Metall. Trans. A, 22(1991), No. 10, p. 2211.
S. Kawanishi, T. Yoshikawa, and T. Tanaka, Equilibrium phase relationship between SiC and a liquid phase in the Fe-Si-C system at 1523-1723 K, Mater. Trans., 50(2009), No. 4, p. 806.
A. Klimczyk, R. Stachura, M. Bernasowski, and A. Ledzki, Silicon behaviour at the blast furnace process, J. Achiev. Mater. Manuf. Eng., 55(2012), No. 2, p. 712.
R. Khanna, F. McCarthy, H.P. Sun, N. Simento, and V. Sahajwalla, Dissolution of carbon from coal-chars into liquid iron at 1550 °C, Metall. Mater. Trans. B, 36(2005), No. 6, p. 719.
B. McDonald, C. Wu, V. Sahajwalla, K. Farrell, and T. Wall, Dissolution studies of carbonaceous materials in blast furnace hot metal during pulverised coal injection, [in] 57th ICSTI / Ironmaking Conference Proceedings, Iron and Steel Society, Toronto, Canada, 1998, p. 1889.
M.W. Chapman, B.J. Monaghan, S.A. Nightingale, J.G. Mathieson, and R.J. Nightingale, Formation of a mineral layer during coke dissolution into liquid iron and its influence on the kinetics of coke dissolution rate, Metall. Mater. Trans. B, 39(2008), No. 3, p. 418.
S.T. Cham, V. Sahajwalla, R. Sakurovs, H.P. Sun, and M. Dubikova, Factors influencing carbon dissolution from cokes into liquid iron, ISIJ Int., 44(2004), No. 11, p. 1835.
F. McCarthy, V. Sahajwalla, J. Hart, and N. Saha-Chaudhury, Influence of ash on interfacial reactions between coke and liquid iron, Metall. Mater. Trans. B, 34(2003), No. 5, p. 573.
S.S. Gornostayev and J.J. Härkki, Graphite crystals in blast furnace coke, Carbon, 45(2007), No. 6, p. 1145.
S.S. Gornostayev, T.M.J. Fabritius, O. Kerkkonen, and J.J. Härkki, Fe-Si droplets associated with graphite on blast furnace coke, Int. J. Miner. Metall. Mater., 19(2012), No. 6, p. 478.
O. Kerkkonen, Tuyere drilling coke sample data from Rautaruukki’s blast furnaces No.1 and 2, [in] Proceedings of AISTech2004 Iron & Steel Technology Conference, Nashville, 2004, p. 469.
T. Kokkonen, S. Gornostayev., and T. Fabritius, Preparation of samples of metallurgical coke for solid-state analysis, [in] Abstracts of 62nd Meeting of the Scandinavian Microscopy Society, Oulu, 2011, p. 91.
S.M. Winder, D.X. Liu, and J.W. Bender, Synthesis and characterization of compound-curved graphite, Carbon, 44(2006), No. 14, p. 3037.
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R. Ben Mahfoud, J. Melançon, A.D. Pelton, and S. Petersen, FactSage thermochemical software and databases, Calphad, 26(2002), No. 2, p. 189.
G. Quinn, B. Faraj, R. Callcott, and T. Callcott, Elucidation of the effects of minerals on coke behaviour in the blast furnace, [in] ACARP Project C10054, Brisbane, 2002, p. 23.
S.S. Gornostayev, O. Kerkkonen, and J.J. Härkki, Importance of mineralogical data for influencing properties of coke: a reference on SiO2 polymorphs, Steel Res. Int., 77(2006), No. 11, p. 770.
C. Yamagata, Y. Kajiwara, and S. Suyama, Desiliconization reaction of pig iron with MnO containing blast furnace slag and coke-coexisting condition SiO2 in coke ash under operating condition in blast furnace, Tetsu-to-Hagané, 73(1987), p. 186.
J.F. Elliot, M. Gleiser, and V. Ramakrishna, Thermochemistry for Steelmaking, Addison-Wesley, London, 1963, p. 485.
R.D. Kamenev, V.L. Shatlov, I.A. Prokof'ev, E.G. Donskov, and V.I. Bondarenko, Expediency of blast-furnace smelting of cast iron on a charge containing free quartz, Metallurgist, 22(1978), No. 2, p. 89.
P.J. Heaney, Structure and chemistry of the low-pressure silica polymorphs, Rev. Mineral., 29(1994), p. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gornostayev, S.S., Heikkinen, EP., Heino, J.J. et al. Fe-Si particles on the surface of blast furnace coke. Int J Miner Metall Mater 22, 697–703 (2015). https://doi.org/10.1007/s12613-015-1124-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-015-1124-9