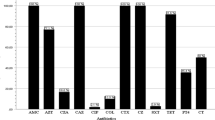
Abstract
Bifidobacteria are commonly used as probiotics in the food industry. The resistance of Bifidobacterium species to antibiotics is closely linked to food safety. However, we still lack a system for the safety evaluation of antibiotic resistance in bifidobacteria, and genus-level microbiological cut-off values remain in use for the determination of phenotypic resistance of Bifidobacterium strains to a given antibiotic. Here, we collected a total of 422 gut-derived bifidobacterial strains isolated from Chinese population and identified their phenotypic resistance profiles against ampicillin, amoxicillin, ciprofloxacin, chloramphenicol, clindamycin, erythromycin, rifampicin, tetracycline, trimethoprim, and vancomycin. Different Bifidobacterium species were found to have varying tolerances to the same antibiotic; therefore, we further established species-specific cut-off values for bifidobacterial species to ten antibiotics. Species-specific rather than genus-specific cut-off values for species belonging to the same taxon were considered more suitable to determine the phenotypic resistance of a Bifidobacterium strain. Moreover, a comprehensive scanning of antibiotic resistance genes in all Bifidobacterium strains tested revealed that the existence of the tetracycline resistance gene tet(W) and the erythromycin/clindamycin resistance gene ErmX is closely related to host phenotypes. Our findings provide guidance and reference values at both phenotype and genotype levels for the safe application of bifidobacteria in the food industry and the development of probiotic resistance evaluation standards.



Similar content being viewed by others
Data Availability
All 422 Bifidobacterium genomes involved in this study were deposited into the NCBI Sequence Read Archive database under the BioProject (No. PRJNA681061), and the specific accession numbers are listed in Supplementary Table S1. Other data will be made available on request.
References
O’Callaghan A, van Sinderen D (2016) Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 7:925. https://doi.org/10.3389/fmicb.2016.00925
Vitellio P, Celano G, Bonfrate L, Gobbetti M, Portincasa P, De Angelis M (2019) Effects of Bifidobacterium longum and Lactobacillus rhamnosus on gut microbiota in patients with lactose intolerance and persisting functional gastrointestinal symptoms: a randomised, double-blind, cross-over study. Nutrients 11:886. https://doi.org/10.3390/nu11040886
Delcaru C, Alexandru I, Podgoreanu P, Cristea VC, Bleotu C, Chifiriuc MC, Bezirtzoglou E, Lazar V (2016) Antagonistic activities of some Bifidobacterium sp. strains isolated from resident infant gastrointestinal microbiota on Gram-negative enteric pathogens. Anaerobe 39:39–44. https://doi.org/10.1016/j.anaerobe.2016.02.010
Medina M, Izquierdo E, Ennahar S, Sanz Y (2007) Differential immunomodulatory properties of Bifidobacterium longum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol 150:531–538. https://doi.org/10.1111/j.1365-2249.2007.03522.x
Makizaki Y, Maeda A, Oikawa Y, Tamura S, Tanaka Y, Nakajima S, Yamamura H (2019) Alleviation of low-fiber diet-induced constipation by probiotic Bifidobacterium bifidum G9–1 is based on correction of gut microbiota dysbiosis. Biosci Microb Food H 38:49–53. https://doi.org/10.12938/bmfh.18-020
Minami J, Iwabuchi N, Tanaka M, Yamauchi K, Xiao JZ, Abe F, Sakane N (2018) Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: a randomized, double-blind, placebo-controlled trial. Biosci Microb Food H 37:67–75. https://doi.org/10.12938/bmfh.18-001
Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, Tobin JM, Wilkinson S, Kothari A, Morrison M, O’Rourke P, Pelecanos A, Dekker Nitert M (2019) Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care 42:364–371. https://doi.org/10.2337/dc18-2248
Fahmy CA, Gamal-Eldeen AM, El-Hussieny EA, Raafat BM, Mehanna NS, Talaat RM, Shaaban MT (2019) Bifidobacterium longum suppresses murine colorectal cancer through the modulation of oncomiRs and tumor suppressor miRNAs. Nutr Cancer 71:688–700. https://doi.org/10.1080/01635581.2019.1577984
EFSA Biohaz Panel (EFSA Panel on Biological Hazards) (2015) Scientific opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA J 11:3449. https://doi.org/10.2903/j.efsa.2013.3449
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (2012) Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10:2740. https://doi.org/10.2903/j.efsa.2012.2740
Berendonk TU, Manaia CM, Merlin C, Fatta-Kassinos D, Cytryn E, Walsh F, Bürgmann H, Sørum H, Norström M, Pons MN, Kreuzinger N, Huovinen P, Stefani S, Schwartz T, Kisand V, Baquero F, Martinez JL (2015) Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol 13:310–317. https://doi.org/10.1038/nrmicro3439
Hu Y, Yang X, Qin J, Lu N, Cheng G, Wu N, Pan Y, Li J, Zhu L, Wang X, Meng Z, Zhao F, Liu D, Ma J, Qin N, Xiang C, Xiao Y, Li L, Yang H, Wang J, Yang R, Gao GF, Wang J, Zhu B (2013) Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat Commun 4:2151. https://doi.org/10.1038/ncomms3151
Pei Z, Sadiq FA, Han X, Zhao J, Zhang H, Ross RP, Lu W, Chen W (2021) Comprehensive scanning of prophages in Lactobacillus: distribution, diversity, antibiotic resistance genes, and linkages with CRISPR-Cas systems. mSystems 6:e0121120. https://doi.org/10.1128/mSystems.01211-20
Kushiro A, Chervaux C, Cools-Portier S, Perony A, Legrain-Raspaud S, Obis D, Onoue M, van de Moer A (2009) Antimicrobial susceptibility testing of lactic acid bacteria and bifidobacteria by broth microdilution method and Etest. Int J Food Microbiol 132:54–58. https://doi.org/10.1016/j.ijfoodmicro.2009.03.012
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79. https://doi.org/10.1016/j.jpha.2015.11.005
EUCAST (European Committee on Antimicrobial Susceptibility Testing) (2021) SOP 1.4 Setting breakpoints for new antimicrobial agents. Retrieved from https://www.eucast.org/eucastsops/. Accessed 11 Oct 2022
EUCAST (European Committee on Antimicrobial Susceptibility Testing) (2021) MIC distributions and the setting of epidemiological cutoff (ECOFF) values. Retrieved from https://www.eucast.org/eucastsops/. Accessed 11 Oct 2022
Klare I, Konstabel C, Werner G, Huys G, Vankerckhoven V, Kahlmeter G, Hildebrandt B, Müller-Bertling S, Witte W, Goossens H (2007) Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J Antimicrob Chemoth 59:900–912. https://doi.org/10.1093/jac/dkm035
ISO (International Organization for Standardization) (2010) Milk and milk products—determination of the minimal inhibitory concentration (MIC) of antibiotics applicable to bifidobacteria and non-enterococcal lactic acid bacteria (LAB). ISO 10932:2010, (IDF 223:2010). ISO, Geneva, Switzerland
Turnidge J, Kahlmeter G, Kronvall G (2006) Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infec 12:418–425. https://doi.org/10.1111/j.1469-0691.2006.01377.x
Kronvall G (2010) Normalized resistance interpretation as a tool for establishing epidemiological MIC susceptibility breakpoints. J Clin Microbiol 48:4445–4452. https://doi.org/10.1128/jcm.01101-10
Smith P, Finnegan W, Ngo T, Kronvall G (2018) Influence of incubation temperature and time on the precision of MIC and disc diffusion antimicrobial susceptibility test data. Aquaculture 490:19–24. https://doi.org/10.1016/j.aquaculture.2018.02.020
Cafarchia C, Iatta R, Immediato D, Puttilli MR, Otranto D (2015) Azole susceptibility of Malassezia pachydermatis and Malassezia furfur and tentative epidemiological cut-off values. Med Mycol 53:743–748. https://doi.org/10.1093/mmy/myv049
Xie Y, Wu G, Tang J, Luo R, Patterson J, Liu S, Huang W, He G, Gu S, Li S, Zhou X, Lam TW, Li Y, Xu X, Wong GK, Wang J (2014) SOAPdenovo-Trans: de novo transcriptome assembly with short RNA-Seq reads. Bioinformatics 30:1660–1666. https://doi.org/10.1093/bioinformatics/btu077
Xu M, Guo L, Gu S, Wang O, Zhang R, Peters BA, Fan G, Liu X, Xu X, Deng L, Zhang Y (2020) TGS-GapCloser: a fast and accurate gap closer for large genomes with low coverage of error-prone long reads. Giga Sci 9:giaa094. https://doi.org/10.1093/gigascience/giaa094
Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ (2010) Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. https://doi.org/10.1186/1471-2105-11-119
Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Cheng NAV, AA, Liu S, Min SY, Miroshnichenko A, Tran HK, Werfalli RE, Nasir JA, Oloni M, Speicher DJ, Florescu A, Singh B, Faltyn M, Hernandez-Koutoucheva A, Sharma AN, Bordeleau E, Pawlowski AC, Zubyk HL, Dooley D, Griffiths E, Maguire F, Winsor GL, Beiko RG, Brinkman FSL, Hsiao WWL, Domselaar GV, McArthur AG (2020) CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 48:D517–D525. https://doi.org/10.1093/nar/gkz935
Ouoba LI, Lei V, Jensen LB (2008) Resistance of potential probiotic lactic acid bacteria and bifidobacteria of African and European origin to antimicrobials: determination and transferability of the resistance genes to other bacteria. Int J Food Microbiol 121:217–224. https://doi.org/10.1016/j.ijfoodmicro.2007.11.018
Lokesh D, Parkesh R, Kammara R (2018) Bifidobacterium adolescentis is intrinsically resistant to antitubercular drugs. Sci Rep 8:11897. https://doi.org/10.1038/s41598-018-30429-2
Papadopoulos P, Papadopoulos T, Angelidis AS, Boukouvala E, Zdragas A, Papa A, Hadjichristodoulou C, Sergelidis D (2018) Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus (MRSA) along the production chain of dairy products in north-western Greece. Food Microbiol 69:43–50. https://doi.org/10.1016/j.fm.2017.07.016
Karampatakis T, Antachopoulos C, Tsakris A, Roilides E (2017) Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in Greece: an extended review (2000–2015). Future Microbiol 12:801–815. https://doi.org/10.2217/fmb-2016-0200
Ullah W, Qasim M, Rahman H, Bari F, Khan S, Rehman ZU, Khan Z, Dworeck T, Muhammad N (2016) Multi drug resistant Pseudomonas aeruginosa: pathogen burden and associated antibiogram in a tertiary care hospital of Pakistan. Microb Pathog 97:209–212. https://doi.org/10.1016/j.micpath.2016.06.017
Kimura A, Yossapol M, Shibata S, Asai T (2017) Selection of broad-spectrum cephalosporin-resistant Escherichia coli in the feces of healthy dogs after administration of first-generation cephalosporins. Microbiol Immunol 61:34–41. https://doi.org/10.1111/1348-0421.12466
Van Rossum T, Ferretti P, Maistrenko OM, Bork P (2020) Diversity within species: interpreting strains in microbiomes. Nat Rev Microbiol 18:491–506. https://doi.org/10.1038/s41579-020-0368-1
Ge B, Wang F, Sjölund-Karlsson M, McDermott PF (2013) Antimicrobial resistance in Campylobacter: susceptibility testing methods and resistance trends. J Microbiol Meth 95:57–67. https://doi.org/10.1016/j.mimet.2013.06.021
Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Jones RN, Turnidge J, Diekema DJ (2010) Wild-type MIC distributions and epidemiological cutoff values for the echinocandins and Candida spp. J Clin Microbiol 48:52–56. https://doi.org/10.1128/jcm.01590-09
Lopez B, Siqueira de Oliveira R, Pinhata JMW, Chimara E, Pacheco Ascencio E, Puyén Guerra ZM, Wainmayer I, Simboli N, Del Granado M, Palomino JC, Ritacco V, Martin A (2019) Bedaquiline and linezolid MIC distributions and epidemiological cut-off values for Mycobacterium tuberculosis in the Latin American region. J Antimicrob Chemoth 74:373–379. https://doi.org/10.1093/jac/dky414
Verraes C, Van Boxstael S, Van Meervenne E, Van Coillie E, Butaye P, Catry B, de Schaetzen MA, Van Huffel X, Imberechts H, Dierick K, Daube G, Saegerman C, De Block J, Dewulf J, Herman L (2013) Antimicrobial resistance in the food chain: a review. Int J Env Res Pub He 10:2643–2669. https://doi.org/10.3390/ijerph10072643
Founou LL, Founou RC, Essack SY (2016) Antibiotic resistance in the food chain: a developing country-perspective. Front Microbiol 7:1881. https://doi.org/10.3389/fmicb.2016.01881
Masco L, Van Hoorde K, De Brandt E, Swings J, Huys G (2006) Antimicrobial susceptibility of Bifidobacterium strains from humans, animals and probiotic products. J Antimicrob Chemoth 58:85–94. https://doi.org/10.1093/jac/dkl197
Xiao JZ, Takahashi S, Odamaki T, Yaeshima T, Iwatsuki K (2010) Antibiotic susceptibility of bifidobacterial strains distributed in the Japanese market. Biosci Biotech Bioch 74:336–342. https://doi.org/10.1271/bbb.90659
Nolivos S, Cayron J, Dedieu A, Page A, Delolme F, Lesterlin C (2019) Role of AcrAB-TolC multidrug efflux pump in drug-resistance acquisition by plasmid transfer. Science 364:778–782. https://doi.org/10.1126/science.aav6390
Aires J, Doucet-Populaire F, Butel MJ (2007) Tetracycline resistance mediated by tet(W), tet(M), and tet(O) genes of Bifidobacterium isolates from humans. Appl Environ Microbiol 73:2751–2754. https://doi.org/10.1128/aem.02459-06
Gueimonde M, Flórez AB, van Hoek AH, Stuer-Lauridsen B, Strøman P, de los Reyes-Gavilán CG, Margolles A, (2010) Genetic basis of tetracycline resistance in Bifidobacterium animalis subsp. lactis. Appl Environ Microbiol 76:3364–3369. https://doi.org/10.1128/aem.03096-09
Nguyen F, Starosta AL, Arenz S, Sohmen D, Dönhöfer A, Wilson DN (2014) Tetracycline antibiotics and resistance mechanisms. Biol Chem 395:559–575. https://doi.org/10.1515/hsz-2013-0292
Wang N, Hang X, Zhang M, Peng X, Yang H (2017) New genetic environments of the macrolide-lincosamide-streptogramin resistance determinant erm(X) and their influence on potential horizontal transferability in bifidobacteria. Int J Antimicrob Ag 50:572–580. https://doi.org/10.1016/j.ijantimicag.2017.04.007
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol R 74:417–433. https://doi.org/10.1128/mmbr.00016-10
Heuer H, Schmitt H, Smalla K (2011) Antibiotic resistance gene spread due to manure application on agricultural fields. Curr Opin Microbiol 14:236–243. https://doi.org/10.1016/j.mib.2011.04.009
Woegerbauer M, Zeinzinger J, Gottsberger RA, Pascher K, Hufnagl P, Indra A, Fuchs R, Hofrichter J, Kopacka I, Korschineck I, Schleicher C, Schwarz M, Steinwider J, Springer B, Allerberger F, Nielsen KM, Fuchs K (2015) Antibiotic resistance marker genes as environmental pollutants in GMO-pristine agricultural soils in Austria. Environ Pollut 206:342–351. https://doi.org/10.1016/j.envpol.2015.07.028
Adam M, Murali B, Glenn NO, Potter SS (2008) Epigenetic inheritance based evolution of antibiotic resistance in bacteria. BMC Evol Biol 8:52. https://doi.org/10.1186/1471-2148-8-52
Arnold BJ, Huang IT, Hanage WP (2022) Horizontal gene transfer and adaptive evolution in bacteria. Nat Rev Microbiol 20:206–218. https://doi.org/10.1038/s41579-021-00650-4
Liu R, Yang B, Stanton C, Paul Ross R, Zhao J, Zhang H, Chen W (2020) Comparative genomics and gene-trait matching analysis of Bifidobacterium breve from Chinese children. Food Biosci 36:100631. https://doi.org/10.1016/j.fbio.2020.100631
Lu W, Pei Z, Zang M, Lee YK, Zhao J, Chen W, Wang H, Zhang H (2021) Comparative genomic analysis of Bifidobacterium bifidum strains isolated from different niches. Genes (Basel) 12:1054. https://doi.org/10.3390/genes12101504
Jacobsen L, Wilcks A, Hammer K, Huys G, Gevers D, Andersen SR (2007) Horizontal transfer of tet(M) and erm(B) resistance plasmids from food strains of Lactobacillus plantarum to Enterococcus faecalis JH2-2 in the gastrointestinal tract of gnotobiotic rats. FEMS Microbiol Ecol 59:158–166. https://doi.org/10.1111/j.1574-6941.2006.00212.x
Feld L, Schjørring S, Hammer K, Licht TR, Danielsen M, Krogfelt K, Wilcks A (2008) Selective pressure affects transfer and establishment of a Lactobacillus plantarum resistance plasmid in the gastrointestinal environment. J Antimicrob Chemoth 61:845–852. https://doi.org/10.1093/jac/dkn033
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31820103010, 31972085, and 32202059), the International Science and Technology Cooperation Project of Jiangsu Province (Grant No. BZ2019016), 111project (BP0719028), and the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province. We would like to thank Editage (www.editage.com) and Home for Researchers (www.home-for-researchers.com/) for English language editing.
Author information
Authors and Affiliations
Contributions
Zhangming Pei: methodology, investigation, formal analysis, visualization, and writing—original draft. Yufei Liu: visualization and writing—review and editing. Fang Zhao: formal analysis, investigation, and methodology. Hongchao Wang: software and writing—review and editing. Jianxin Zhao: supervision and resources. Wei Chen: supervision and resources. Wenwei Lu: conceptualization, supervision, validation, and resources.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, Z., Liu, Y., Zhao, F. et al. Antibiotic Susceptibility Testing and Establishment of Tentative Species-Specific Microbiological Cut-off Values for Bifidobacteria Isolated from Chinese Population. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10128-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10128-9