Abstract
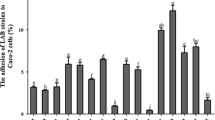
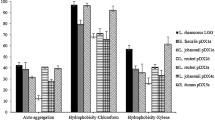
The probiotic properties and inhibitory effect on Salmonella Typhimurium adhesion on human enterocyte-like HT-29-Luc cells of three Lactobacillus plantarum strains isolated from fermented fish, beach sand and a coastal plant were determined. Compared with the type strain L. plantarum NBRC 15891T, which was isolated from pickled cabbage, L. plantarum Tennozu-SU2 isolated from the acorn of a coastal tree showed high autoaggregation in de Man, Rogosa and Sharpe (MRS) broth and an antagonistic effect against S. Typhimurium in brain heart infusion (BHI) broth. Furthermore, heat-killed L. plantarum Tennozu-SU2 cells inhibited S. Typhimurium adhesion on HT-29-Luc cells. Both live and heat-killed L. plantarum Tennozu-SU2 cells showed an inhibitory effect on gut colonisation in BALB/c mice, as assessed by viable Salmonella count in faecal samples and by invasion into liver and spleen tissues. The properties shown in this study suggest that L. plantarum Tennozu-SU2 is useful as a starter and probiotic bacteria in functional food material.




Similar content being viewed by others
References
Galis AM, Marcq C, Marlier D, Portetelle D, Van I, Beckers Y, André T (2013) Control of Salmonella contamination of shell eggs—preharvest and postharvest methods. Comp Rev Food Sci Food Safety 12:155–182
Zaki HMBA, Mohamed HMH, El-Sheriff AMA (2015) Improving the antimicrobial efficacy of organic acids against Salmonella enterica attached to chicken skin using SDS with acceptable sensory quality. LWT-Food Sci Technol 64:558–564
Gkana E, Lianou A, Nychas GJE (2016) Transfer of Salmonella enterica serovar Typhimurium from beef to tomato through kitchen equipment and the efficacy of intermediate decontamination procedures. J Food Prot 79:1252–1258
Henao-Herreño LX, López-Tamayo AM, Ramos-Bonilla JP, Haas CN, Husserl J (2016) Risk of illness with Salmonella due to consumption of raw unwashed vegetables irrigated with water from the Bogotá River. Risk Anal. doi:10.1111/risa.12656
Kuda T, Yazaki T, Takahashi H, Kimura B (2013) Effect of dried and vinegar flavored squid products on acid resistance of Salmonella Typhimurium and Staphylococcus aureus. Food Control 30:569–574
Gorman R, Adley CC (2004) Characterization of Salmonella enterica serotype Typhimurium isolates from human, food, and animal sources in the Republic of Ireland. J Clin Microbiol 42:2314–2316
Hernandez SM, Keel K, Sanchez S, Trees E, Gerner-Smidt P, Adams JK, Cheng Y, Ray A 3rd, Martin G, Presotto A, Ruder MG, Brown J, Blehert DS, Cottrell W, Maurer JJ (2012) Epidemiology of a Salmonella enterica subsp. enterica serovar Typhimurium strain associated with a songbird outbreak. Appl Environ Microbiol 78:7290–7298
Fàbrega A, Vila J (2013) Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev 26:308–341
Kuda T, Shibata G, Takahashi H, Kimura B (2015) Effect of quantity of food residues on resistance to desiccation of food-related pathogens adhered to a stainless steel surface. Food Microbiol 46:234–238
Kuda T, Koyanagi T, Shibata G, Takahashi H, Kimura B (2016) Effect of carrot residue on the desiccation and disinfectant resistances of food related pathogens adhered to a stainless steel surfaces. LWT-Food Sci Technol 74:251–254
Ryan D, Pati NB, Ojha UK, Padhi C, Ray S, Jaiswal S, Singh GP, Mannala GK, Schultze T, Chakraborty T, Suar M (2015) Global transcriptome and mutagenic analyses of the acid tolerance response of Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 81:8054–8065
Leekitcharoenphon P, Henderiksen RS, Hello SL, Well FX, Baggesen DL, Jun SR, Ussery DR, Lund O, Crook DW, Wilson DJ, Aerestrup FM (2016) Global genomic epidemiology of Salmonella enterica serovar Typhimurium DT104. Appl Environ Microbiol 82:2516–2526
Nemoto M, Kuda T, Eda M, Yamakawa H, Takahashi H, Kimura B (2016) Protective effects of mekabu aqueous solution fermented by Lactobacillus plantarum Sanriku-SU7 on human enterocyte-like HT-29-luc cells and DSS-induced murine IBD model. Probiotics Antimicro Prot . doi:10.1007/s12602-016-9226-x in print
Eda M, Kuda T, Kataoka M, Takahashi H, Kimura B (2016) Anti-glycation properties of the aqueous extract solutions of dried algae products harvested and made in the Miura Peninsula, Japan, and effect of lactic acid fermentation on the properties. J Appl Phycol . doi:10.1007/s10811-016-0891-7 in print
Kuda T, Sarengaole TH, Kimura B (2016) Alcohol-brewing properties of acid- and bile-tolerant yeasts co-cultured with lactic acid bacteria isolated from traditional handmade domestic dairy products from Inner Mongolia. LWT-Food Sci Technol 63:62–69
Liu WH, Yang CH, Lin CT, Li SW, Cheng WS, Jiang YP, Wu CC, Chang CH, Tsai YC (2015) Genome architecture of Lactobacillus plantarum PS128, a probiotic strain with potential immunomodulatory activity. Gut Pathogens 7:22
Xie J, Yu Q, Nie S, Fan S, Xiong T, Xie M (2015) Effects of Lactobacillus plantarum NCU116 on intestine mucosal immunity in immunosuppressed mice. J Agric Food Chem 63:10914–10920
Tang Y, Dong W, Wan K, Zhang L, Li C, Zhang L, Liu N (2015) Exopolysaccharide produced by Lactobacillus plantarum induces maturation of dendritic cells in BALB/c mice. PLoS One 10:e0143743
Nishimura M, Ohkawara T, Tetsuka K, Kawasaki Y, Nakagawa R, Satoh Y, Sato Y, Nishihara J (2016) Effects of yogurt containing Lactobacillus plantarum HOKKAIDO on immune function and stress markers. J Tradit Complement Med 6:275–280
Xie J, Nie S, Yu Q, Yin J, Xiong T, Gong D, Xie M (2016) Lactobacillus plantarum NCU116 attenuates cyclophosphamide-induced immunosuppression and regulates Th17/Treg cell immune responses in mice. J Agric Food Chem 64:1291–1297
Castillo NA, Perdigón G, de LeBlanc AM (2011) Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol 11:77
Nakazato G, Paganelli FL, Lago JC, Aoki FH, Mobilon C, Brocchi M, Stehling EG, Silveira WD (2011) Lactobacillus acidophilus decrease Salmonella Typhimurium invasion in vivo. J Food Safety 31:284–289
Chen CY, Tsen HY, Lin CL, Lin CK, Chuang LT, Chen CS, Chiang YC (2013) Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. J Med Microbiol 62:1657–1664
Llana MN, Sarnacki SH, Castañeda MRA, Bernal MI, Giacomodonato BMN, Cerquetti MC (2013) Consumption of Lactobacillus casei fermented milk prevents Salmonella reactive arthritis by modulating IL-23/IL-17 expression. PLoS One 8:e82588
Eom JS, Song J, Choi HS (2015) Protective effects of a novel probiotic strain of Lactobacillus plantarum JSA22 from traditional fermented soybean food against infection by Salmonella enterica serovar Typhimurium. J Microbiol Biotechnol 25:479–491
Kanno T, Kuda T, An C, Takahashi H, Kimura B (2012) Radical scavenging capacities of saba-narezushi, Japanese fermented chub mackerel, and its lactic acid bacteria. LWT-Food Sci Technol 47:25–30
Kuda T, Kawahara M, Nemoto M, Takahashi K, Kimura B (2014) In vitro antioxidant and anti-inflammation properties of lactic acid bacteria isolated from fish intestines and fermented fish from the Sanriku Satoumi region in Japan. Food Res Int 64:248–255
Kawahara M, Nemoto M, Nakata T, Kondo S, Takahashi H, Kimura B, Kuda T (2015) Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice. Int Immunopharmacol 26:295–303
Kuda T, Masuko Y, Kawahara M, Kondo S, Nemoto M, Nakata T, Kataoka M, Takahashi H, Kimura B (2016) Bile acid-lowering properties of Lactobacillus plantarum Sanriku–SU3 isolated from Japanese surfperch fish. Food Biosci 14:41–46
Kuda T, Kataoka M, Nemoto M, Kawahara M, Takahashi H, Kimura B (2016) Isolation of lactic acid bacteria from plants of the coastal Satoumi regions for use as starter cultures in fermented milk and soymilk production. LWT-Food Sci Technol 68:202–207
Kuda T, Noguchi Y, Ono M, Takahashi H, Kimura B, Kamita R, Eto T, Kato M, Kawahara M (2014) In vitro evaluation of the fermentative, antioxidant, and anti-inflammation properties of Lactococcus lactis subsp. lactis BF3 and Leuconostoc mesenteroides subsp. mesenteroides BF7 isolated from Oncorhynchus keta intestines in Rausu, Japan. J Funct Foods 11:269–277
Kos B, Šušković J, Vuković S, Šimpraga M, Frece J, Matošić S (2003) Adhesion and aggregation ability of probiotic strain. Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987
Kuda T, Kosaka M, Hirano S, Kawahara M, Sato M, Kaneshima T, Nishizawa M, Takahashi H, Kimura B (2015) Effect of sodium-alginate and laminaran on Salmonella Typhimurium infection in human enterocyte-like HT-29-Luc cells and BALB/c mice. Carbohydr Polymers 125:113–110
Kuda T, Tsuda N, Yano T (2004) Thermal inactivation characteristics of acid and alkaline phosphatase in fish and shellfish. Food Chem 88:543–548
Dimitrov Z, Gotova I, Chorbadjiyska E (2014) In vitro characterization of the adhesive factors of selected probiotics to Caco-2 epithelium cell line. Biotechnol Biotechnol Equip 28:1079–1083
Nakamura S, Kuda T, An C, Kanno T, Takahashi H, Kimura B (2012) Inhibitory effects of Leuconostoc mesenteroides 1RM3 isolated from narezushi, a fermented fish with rice, on Listeria monocytogenes infection to Caco-2 cells and A/J mice. Anaerobe 18:19–24
Giraud E, Lelong B, Raimbaut M (1991) Influence of pH and initial lactate concentration on the growth of Lactobacillus plantarum. Appl Microbiol Biotechnol 36:96–99
Frece J, Kos B, Svetec IK, Zgaga Z, Beganović J, Lebos A, Susković J (2009) Synbiotic effect of Lactobacillus helveticus M92 and prebiotics on the intestinal microflora and immune system of mice. J Dairy Res 76:98–104
Dobson A, Catter PD, Ross PR, Hill C (2012) Bacteriocin production: a probiotic trait? Appl Environ Microbiol 78:1–6
Abedi D, Feizizadef S, Akbari V, Jafarian-Dehkordi A (2013) In vitro anti-bacterial and anti-adherence effects of Lactobacillus delbrueckii subsp bulgaricus on Escherichia coli. Res Pharm Sci 8:260–268
Gagnon M, Berner AZ, Chervet N, Chassard C, Lacroix C (2013) Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J Microbiol Methods 94:274–279
Liu HY, Rooms S, Jonsson H, Ahi D, Dicksved J, Lindberg JE (2015) Effects of Lactobacillus johnsonii and Lactobacillus reuteri on gut barrier function and heat shock proteins in intestinal porcine epithelial cells. Physiol Rep 3:e12355
Drago-Serrano ME, Rivera-Aguilar V, Reséndiz-Albor AA, Campos-Rodríguez R (2010) Lactoferrin increases both resistance to Salmonella typhimurium infection and the production of antibodies in mice. Immun Lett 134:35–46
Acknowledgements
The present study was supported in part by the Terrada Warehouse Company, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal experiments were performed in compliance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions, under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, and approved by the animal experiment committee of Tokyo University of Marine Science and Technology (approval no. H28-4).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hirano, S., Yokota, Y., Eda, M. et al. Effect of Lactobacillus plantarum Tennozu-SU2 on Salmonella Typhimurium Infection in Human Enterocyte-Like HT-29-Luc Cells and BALB/c Mice. Probiotics & Antimicro. Prot. 9, 64–70 (2017). https://doi.org/10.1007/s12602-016-9243-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-016-9243-9