Abstract
Introduction
FCN-159 is a novel, oral, potent, selective MEK1/2 inhibitor in clinical development for the treatment of NRAS-mutant advanced melanoma and neurofibromatosis type 1. We investigated the effect of food on the pharmacokinetics (PK), safety, and tolerability of FCN-159.
Methods
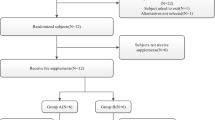
In this single-center, open-label, phase 1 study with a three-period, three-sequence, crossover design, healthy Chinese male subjects (n = 24) were randomized (1:1:1) to receive a single, oral 8 mg dose of FCN-159 in the fasted state (overnight, > 10 h), and with a low-fat and a high-fat meal, separated by a 10-day washout. PK parameters including time to maximum plasma concentration (Cmax) and area under the concentration–time curve (AUC) were compared using geometric least-squares mean ratios (GLSMR), with the fasted state as the reference. A 90% CI for the GLSMR within 80–125% indicated no significant food effect.
Results
A low-fat meal (n = 23) did not affect the PK profile of FCN-159: G LSMR for AUC from time 0 to t (AUC0–t), 106.9% (90% CI 99.9–114.4%); AUC from time 0 to infinity (AUC0–∞), 106.8% (90% CI 100.0–114.0%); Cmax, 96.4% (90% CI 83.9–110.8%). A high-fat meal (n = 24) did not affect exposure to FCN-159 (GLSMR for AUC0–t, 99.4%; 90% CI 99.0–106.3%; AUC0–∞, 99.5 5%; 90% CI 93.2–106.1%), but modestly reduced Cmax by 15% (GLSMR 84.9%; 90% CI 74.0–97.3%). Both the low-fat and high-fat meals slightly prolonged the median time to Cmax by 0.5 h (90% CI 0.5–1.0 h). FCN-159 was generally well tolerated, with a lower incidence of treatment-emergent adverse events following administration in the fasted state than with a low-fat or high-fat meal (20.8%, 39.1%, and 37.5%, respectively).
Conclusion
Food did not affect the PK profile of FCN-159 to a clinically meaningful extent compared with administration in the fasted state.

Similar content being viewed by others
References
Yaeger R, Corcoran RB. Targeting Alterations in the RAF-MEK Pathway. Cancer Discov. 2019;9(3):329–41. https://doi.org/10.1158/2159-8290.CD-18-1321.
Linares MA, Zakaria A, Nizran P. Skin cancer. Prim Care. 2015;42(4):645–59. https://doi.org/10.1016/j.pop.2015.07.006.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54. https://doi.org/10.1038/nature00766.
BioPharma A. Mektovi (binimetinib) US Prescribing Information (January 2019) [June 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210498s001lbl.pdf.
Genentech. Cotelli (cobimetinib) US Prescribing Information (January 2018). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206192s002lbl.pdf.
Novartis. Mekinist (trametinib) US Prescribing Information (January 2022) [June 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/204114s021lbl.pdf.
Novartis. Mekinist (trametinib) EU Summary of Product Characteristics (May 2022). https://www.ema.europa.eu/en/documents/product-information/mekinist-epar-product-information_en.pdf.
Pierre Fabre Medicament. Mektovi (binimetinib) EU Summary of Product Characteristics (January 2022). [June 2022]. https://www.ema.europa.eu/en/documents/product-information/mektovi-epar-product-information_en.pdf.
Roche. Cotellic (cobimetinib) EU Summary of Product Characteristics (November 2021). https://www.ema.europa.eu/en/documents/product-information/cotellic-epar-product-information_en.pdf.
Galvin R, Watson AL, Largaespada DA, Ratner N, Osum S, Moertel CL. Neurofibromatosis in the era of precision medicine: development of MEK inhibitors and recent successes with selumetinib. Curr Oncol Rep. 2021;23(4):45. https://doi.org/10.1007/s11912-021-01032-y.
Anderson JL, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol. 2015;132:75–86. https://doi.org/10.1016/B978-0-444-62702-5.00004-4.
AstraZeneca. Kosulego (selumetinib) US Prescribing Information (December 2021) [June 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213756Orig1s003lbl.pdf.
AstraZeneca. Kosulego (selumetinib) EU Summary of Product Characteristics (July 2022) [cited July 2022]. https://www.ema.europa.eu/en/documents/product-information/koselugo-epar-product-information_en.pdf.
Lin S, Zhao X, Zhou Z, Tan H, Chen L, Tan R, et al. Abstract 1951: FCN-159: a novel, potent and selective oral inhibitor of MEK1/2 for the treatment of solid tumors. Cancer Res. 2020;80(16_Suppl):1951. https://doi.org/10.1158/1538-7445.Am2020-1951.
Si L, Mao L, Zhou L, Li C, Wang X, Cui C, et al. A phase Ia/Ib clinical study to evaluate the safety, pharmacokinetics (PK) and preliminary anti-tumour activity of FCN-159 in patients with advanced melanoma harboring NRAS-aberrant (Ia) and NRAS-mutation (Ib). Ann Oncol. 2019;30(Suppl 5):V562.
Fosun Pharmaceuticals. Data on File 2022.
Mao L, Guo J, Zhu L, Jiang Y, Yan W, Zhang J, et al. FCN-159, a MEK1/2 inhibitor, in patients with advanced melanoma harboring NRAS mutations: a phase 1A dose-escalation study. Cancer Res. 2022;82(12 Suppl):1–6403.
Hu X, Zeng K, Xu Z, Li W, Li C, Kang Z, et al. A multicenter, open-label, single-arm, phase 1 dose-escalation study to evaluate the safety, tolerability, and anti-tumor activity of FCN-159 in adults with neurofibromatosis type 1. J Clin Oncol. 2022;40(16 Suppl):3011. https://doi.org/10.1200/JCO.2022.40.16_suppl.3011.
Cox DS, Papadopoulos K, Fang L, Bauman J, LoRusso P, Tolcher A, et al. Evaluation of the effects of food on the single-dose pharmacokinetics of trametinib, a first-in-class MEK inhibitor, in patients with cancer. J Clin Pharmacol. 2013;53(9):946–54. https://doi.org/10.1002/jcph.115.
Tomkinson H, McBride E, Martin P, Lisbon E, Dymond AW, Cantarini M, et al. Comparison of the pharmacokinetics of the phase II and phase III capsule formulations of selumetinib and the effects of food on exposure: results from two randomized crossover trials in healthy male subjects. Clin Ther. 2017;39(11):2260−75 e1. https://doi.org/10.1016/j.clinthera.2017.08.022.
Cohen-Rabbie S, Mattinson A, So K, Wang N, Goldwater R. Effect of food on capsule and granule formulations of selumetinib. Clin Transl Sci. 2022;15(4):878–88. https://doi.org/10.1111/cts.13209.
AstraZeneca. Koselugo® (selumetinib) US Prescribing Information (December 2021). 2021 [July 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213756Orig1s003lbl.pdf.
Musib L, Choo E, Deng Y, Eppler S, Rooney I, Chan IT, et al. Absolute bioavailability and effect of formulation change, food, or elevated pH with rabeprazole on cobimetinib absorption in healthy subjects. Mol Pharm. 2013;10(11):4046–54. https://doi.org/10.1021/mp400383x.
Center for Drug Evaluation and Research UFaDA. Assessing the effects of food on drugs in INDs and NDAs—clinical pharmacology considerations guidance for industry (2019).
US Food and Drug Administration. Bioavailability Studies Submitted in NDAs or INDs—General Considerations. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioavailability-studies-submitted-ndas-or-inds-general-considerations. Accessed October 2022.
Acknowledgements
Funding
This study was funded by Shanghai Fosun Pharmaceutical Development Co., Ltd. The study sponsor also funded the journal’s Rapid Service fees.
Medical Writing, Editorial, and Other Assistance
Editorial support for this manuscript was provided by Jake Burrell PhD (Rude Health Consulting Ltd.) and paid for by Fosun Pharma.
Author Contributions
Xuhong Wang, Lei Diao, Jiangfan Li, Yan Tan, Kexin Li, Yan Tan, Ai-Min Hui, Zhuli Wu, and Pu Han contributed to the study design, study conduct, and the critical review of the intellectual content of the article, and approved the final version for publication. Zheng Wei and Jingjun Qiu contributed to the analysis and interpretation of data, the critical review of the intellectual content of the article, and approved the final version for publication.
Disclosures
Yan Tan, Zhuli Wu, Pu Han, Zhen Wei, Jingjun Qiu, and Lei Diao are employees of Beijing Fosun Pharmaceutical Research and Development Co., Ltd. Ai-Min Hui is an employee of Fosun Pharma USA Inc. The other authors declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
The study protocol was approved by the Ethics Review Board at Beijing Luhe Hospital, Capital Medical University, Beijing, China (approval number: 2021-LHYW-062-01). The study was conducted in compliance with applicable laws in China, and in accordance with the Chinese Guidelines for Good Clinical Practice and the principles outlined in the Declaration of Helsinki of 1964 and subsequent revisions. All subjects provided written, informed consent prior to participation. This paper was written following the ClinPK guidelines.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Tan, Y., Li, K. et al. Effect of Food (Low and High Fat) on Pharmacokinetics of FCN-159, a Selective MEK Inhibitor, in Healthy Chinese Males. Adv Ther 40, 1074–1086 (2023). https://doi.org/10.1007/s12325-022-02375-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02375-z