Abstract
Somatic symp tom disorders (SSDs) are a group of psychiatric disorders characterized by persistent disproportionate concern and obsessive behaviors regarding physical conditions. Currently, SSDs lack effective treatments and their pathophysiology is unclear. In this paper, we aimed to examine microstructural abnormalities in the brains of patients with SSD using diffusion kurtosis imaging (DKI) and to investigate the correlation between these abnormalities and clinical indicators. Diffusion kurtosis images were acquired from 30 patients with SSD and 30 healthy controls (HCs). Whole-brain maps of multiple diffusion measures, including fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD), mean kurtosis (MK), radial kurtosis (RK), and axial kurtosis (AK), were calculated. To analyze differences between the two groups, nonparametric permutation testing with 10,000 randomized permutations and threshold-free cluster enhancement was used with family-wise error-corrected p values < 0.05 as the threshold for statistical significance. Then, the correlations between significant changes in these diffusion measures and clinical factors were examined. Compared to HCs, patients with SSD had significantly higher FA, MK, and RK and significantly lower MD and RD in the cerebellum, thalamus, basal ganglia, and limbic cortex. The FA in the left caudate and the pontine crossing tract were negatively correlated with disease duration; the MD and the RD in the genu of the corpus callosum were positively correlated with disease duration. Our findings highlight the role of the cerebellum-thalamus-basal ganglia-limbic cortex pathway, especially the cerebellum, in SSDs and enhance our understanding of the pathogenesis of SSDs.

Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington (DC): American Psychiatric Association; 2013.
Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20:23–31.
Boeckle M, Schrimpf M, Liegl G, Pieh C. Neural correlates of somatoform disorders from a meta-analytic perspective on neuroimaging studies. Neuroimage Clin. 2016;11:606–13. https://doi.org/10.1016/j.nicl.2016.04.001.
Hakala M, Karlsson H, Kurki T, Aalto S, Koponen S, Vahlberg T, Niemi PM. Volumes of the caudate nuclei in women with somatization disorder and healthy women. Psychiatry Res. 2004;131:71–8. https://doi.org/10.1016/j.pscychresns.2004.03.001.
Begue I, Adams C, Stone J, Perez DL. Structural alterations in functional neurological disorder and related conditions: a software and hardware problem? Neuroimage Clin. 2019;22:101798. https://doi.org/10.1016/j.nicl.2019.101798.
Delvecchio G, Rossetti MG, Caletti E, Arighi A, Galimberti D, Basilico P, Mercurio M, Paoli R, Cinnante C, Triulzi F, Altamura AC, Scarpini E, Brambilla P. The neuroanatomy of somatoform disorders: a magnetic resonance imaging study. Psychosomatics. 2019;60:278–88. https://doi.org/10.1016/j.psym.2018.07.005.
Liang HB, Dong L, Cui Y, Wu J, Tang W, Du X, Liu JR. Significant structural alterations and functional connectivity alterations of cerebellar gray matter in patients with somatic symptom disorder. Front Neurosci. 2022;16:816435. https://doi.org/10.3389/fnins.2022.816435.
Park HY, Jang YE, Sunwoo L, Yoon IY, Park B. A longitudinal study on attenuated structural covariance in patients with somatic symptom disorder. Front Psychiatry. 2022;13:817527. https://doi.org/10.3389/fpsyt.2022.817527.
Zhao J, Su Q, Liu F, Zhang Z, Li R, Zhu F, Wu R, Zhao J, Guo W. Regional white matter volume abnormalities in first-episode somatization disorder. Int J Psychophysiol. 2018;133:12–6. https://doi.org/10.1016/j.ijpsycho.2018.09.003.
Diez I, Williams B, Kubicki MR, Makris N, Perez DL. Reduced limbic microstructural integrity in functional neurological disorder. Psychol Med. 2021;51:485–93. https://doi.org/10.1017/S0033291719003386.
Sone D, Sato N, Ota M, Kimura Y, Matsuda H. Widely impaired white matter integrity and altered structural brain networks in psychogenic non-epileptic seizures. Neuropsychiatr Dis Treat. 2019;15:3549–55. https://doi.org/10.2147/NDT.S235159.
Lee S, Allendorfer JB, Gaston TE, Griffis JC, Hernando KA, Knowlton RC, Szaflarski JP, Ver Hoef LW. White matter diffusion abnormalities in patients with psychogenic non-epileptic seizures. Brain Res. 2015;1620:169–76. https://doi.org/10.1016/j.brainres.2015.04.050.
Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Marien P, Molinari M, Moulton E, Orsi L, Van Overwalle F, Papadelis C, Priori A, Sacchetti B, Schutter DJ, Styliadis C, Verhoeven J. Consensus paper: cerebellum and emotion. Cerebellum. 2017;16:552–76. https://doi.org/10.1007/s12311-016-0815-8.
Phillips JR, Hewedi DH, Eissa AM, Moustafa AA. The cerebellum and psychiatric disorders. Front Public Health. 2015;3:66. https://doi.org/10.3389/fpubh.2015.00066.
Moreno-Rius J. The cerebellum under stress. Front Neuroendocrinol. 2019;54:100774. https://doi.org/10.1016/j.yfrne.2019.100774.
Pierce JE, Peron J. The basal ganglia and the cerebellum in human emotion. Soc Cogn Affect Neurosci. 2020;15:599–613. https://doi.org/10.1093/scan/nsaa076.
Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci. 2018;19:338–50. https://doi.org/10.1038/s41583-018-0002-7.
Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–6. https://doi.org/10.1073/pnas.1000496107.
Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–3. https://doi.org/10.1038/nn1544.
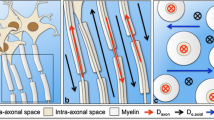
Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23:698–710. https://doi.org/10.1002/nbm.1518.
Tan Y, Zhang H, Wang X, Qin J, Wang L, Yang G, Yan H. Comparing the value of DKI and DTI in detecting isocitrate dehydrogenase genotype of astrocytomas. Clin Radiol. 2019;74:314–20. https://doi.org/10.1016/j.crad.2018.12.004.
Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes-what do we know? Front Neurol. 2018;9:92. https://doi.org/10.3389/fneur.2018.00092.
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432–40. https://doi.org/10.1002/mrm.20508.
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97. https://doi.org/10.1016/j.neuroimage.2014.01.060.
Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061.
Rossetti MG, Delvecchio G, Calati R, Perlini C, Bellani M, Brambilla P. Structural neuroimaging of somatoform disorders: a systematic review. Neurosci Biobehav Rev. 2021;122:66–78. https://doi.org/10.1016/j.neubiorev.2020.12.017.
Bonnefil V, Dietz K, Amatruda M, Wentling M, Aubry AV, Dupree JL, Temple G, Park HJ, Burghardt NS, Casaccia P and Liu J. Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. Elife. 2019; 8:e40855. https://doi.org/10.7554/eLife.40855.
Bai Y, Lin Y, Tian J, Shi D, Cheng J, Haacke EM, Hong X, Ma B, Zhou J, Wang M. Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology. 2016;278:496–504. https://doi.org/10.1148/radiol.2015142173.
Delgado y Palacios R, Verhoye M, Henningsen K, Wiborg O, Van der Linden A. Diffusion kurtosis imaging and high-resolution MRI demonstrate structural aberrations of caudate putamen and amygdala after chronic mild stress. PLoS One. 2014;9:e95077. https://doi.org/10.1371/journal.pone.0095077.
Espiridion ED, Kerbel SA. A systematic literature review of the association between somatic symptom disorder and antisocial personality disorder. Cureus. 2020;12:e9318. https://doi.org/10.7759/cureus.9318.
Shi J, Yang S, Wang J, Huang S, Yao Y, Zhang S, Zhu W, Shao J. Detecting normal pediatric brain development with diffusional kurtosis imaging. Eur J Radiol. 2019;120:108690. https://doi.org/10.1016/j.ejrad.2019.108690.
Johnson FK, Kaffman A. Early life stress perturbs the function of microglia in the developing rodent brain: new insights and future challenges. Brain Behav Immun. 2018;69:18–27. https://doi.org/10.1016/j.bbi.2017.06.008.
Jia X, Gao Z, Hu H. Microglia in depression: current perspectives. Sci China Life Sci. 2021;64:911–25. https://doi.org/10.1007/s11427-020-1815-6.
Hughes AN, Appel B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat Neurosci. 2020;23:1055–66. https://doi.org/10.1038/s41593-020-0654-2.
Kennis M, Van Rooij SJ, Tromp do PM, Fox AS, Rademaker AR, Kahn RS, Kalin NH, Geuze E. Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacol. 2015;40:2434–42. https://doi.org/10.1038/npp.2015.94.
McCunn P, Richardson JD, Jetly R, Dunkley B. Diffusion tensor imaging reveals white matter differences in military personnel exposed to trauma with and without post-traumatic stress disorder. Psychiatry Res. 2021;298:113797. https://doi.org/10.1016/j.psychres.2021.113797.
Tae WS, Ham BJ, Pyun SB, Kang SH, Kim BJ. Current clinical applications of diffusion-tensor imaging in neurological disorders. J Clin Neurol. 2018;14:129–40. https://doi.org/10.3988/jcn.2018.14.2.129.
Geeraert BL, Lebel RM, Lebel C. A multiparametric analysis of white matter maturation during late childhood and adolescence. Hum Brain Mapp. 2019;40:4345–56. https://doi.org/10.1002/hbm.24706.
Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, Kolb FP. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review Cortex. 2010;46:845–57. https://doi.org/10.1016/j.cortex.2009.06.009.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–44. https://doi.org/10.1016/j.cortex.2009.11.008.
Moreno-Rius J. The cerebellum in fear and anxiety-related disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:23–32. https://doi.org/10.1016/j.pnpbp.2018.04.002.
Strata P. The emotional cerebellum. Cerebellum. 2015;14:570–7. https://doi.org/10.1007/s12311-015-0649-9.
Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. 2016;233:1637–50. https://doi.org/10.1007/s00213-016-4218-9.
de Greck M, Scheidt L, Bolter AF, Frommer J, Ulrich C, Stockum E, Enzi B, Tempelmann C, Hoffmann T, Han S, Northoff G. Altered brain activity during emotional empathy in somatoform disorder. Hum Brain Mapp. 2012;33:2666–85. https://doi.org/10.1002/hbm.21392.
Liang H, Dong L, Cui Y, Wu J, Tang W, Du X, Liu J. Significant structural alterations and functional connectivity alterations of cerebellar gray matter in patients with somatic symptom disorder. Front Neurosci. 2022;16:816435. https://doi.org/10.3389/fnins.2022.816435.
Torrico TJ and Munakomi S. Neuroanatomy, Thalamus. Treasure Island (FL): StatPearls; 2022.
Hanggi J, Bellwald D, Brugger P. Shape alterations of basal ganglia and thalamus in xenomelia. Neuroimage Clin. 2016;11:760–9. https://doi.org/10.1016/j.nicl.2016.05.015.
He M, Shen Z, Ping L, Zhou C, Cheng Y, Xu X. Age-related heterogeneity revealed by disruption of white matter structural networks in patients with first-episode untreated major depressive disorder: WM Network In OA-MDD. J Affect Disord. 2022;303:286–96. https://doi.org/10.1016/j.jad.2022.02.036.
Perez DL, Barsky AJ, Vago DR, Baslet G, Silbersweig DA. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27:e40-50. https://doi.org/10.1176/appi.neuropsych.13070170.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 81571658 to X. X. Du and grant No. 81271302 to J.R. Liu), a research project of the Shanghai University of Sport (Grant No. 2022XJ002 to X. X. Du), the Shanghai Municipal Science and Technology Commission (Innovation Research Project, No. 14JC1404300 to J.R. Liu), Shanghai Hospital Development Center (Prevention and Control of Chronic Diseases Project, No. SHDC12015310 to J.R. Liu), SHSMU-ION Research Center for Brain Disorders (project No. 2015NKX006 to J.R. Liu), Shanghai Municipal Education Commission (Gaofeng Clinical Medicine Grant Support, project No. 20161422 to J. R. Liu), Shanghai Jiao Tong University School of Medicine (Clinical Research Project, No. DLY201614 to J.R. Liu), and Shanghai Municipal Science and Technology Commission (Biomedicine Key Program, No. 16411953100 to J.R. Liu). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, L., Liang, HB., Du, J. et al. Microstructural Differences of the Cerebellum-Thalamus-Basal Ganglia-Limbic Cortex in Patients with Somatic Symptom Disorders: a Diffusion Kurtosis Imaging Study. Cerebellum 22, 840–851 (2023). https://doi.org/10.1007/s12311-022-01461-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-022-01461-w