Abstract
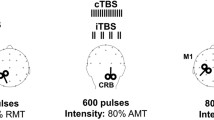
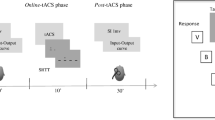
Continuous theta-burst stimulation (cTBS) applied over the cerebellum exerts long-lasting effects by modulating long-term synaptic plasticity, which is thought to be the basis of learning and behavioral adaptation. To investigate the impact of cTBS over the cerebellum on short-term sensory-motor memory, we recorded in two groups of eight healthy subject each the visually guided saccades (VGSs), the memory-guided saccades (MGSs), and the multiple memory-guided saccades (MMGSs), before and after cTBS (cTBS group) or simulated cTBS (control group). In the cTBS group, cTBS determined hypometria of contralateral centrifugal VGSs and worsened the accuracy of MMGS bilaterally. In the control group, no significant differences were found between the two recording sessions. These results indicate that cTBS over the cerebellum causes eye movement effects that last longer than the stimulus duration. The VGS contralateral hypometria suggested that we eventually inhibited the fastigial nucleus on the stimulated side. MMGSs in normal subjects have a better final accuracy with respect to MGSs. Such improvement is due to the availability in MMGSs of the efference copy of the initial reflexive saccade directed toward the same peripheral target, which provides a sensory-motor information that is memorized and then used to improve the accuracy of the subsequent volitional memory-guided saccade. Thus, we hypothesize that cTBS disrupted the capability of the cerebellum to make an internal representation of the memorized sensory-motor information to be used after a short interval for forward control of saccades.



Similar content being viewed by others
References
Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4(5):467–75.
Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78(3–5):272–303.
Ebner TJ, Pasalar S. Cerebellum predicts the future motor state. Cerebellum. 2008;7(4):583–8.
Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14(2):225–32.
Ivry RB et al. The cerebellum and event timing. Ann NY Acad Sci. 2002;978:302–17.
Spencer RM, Ivry RB. Sequence learning is preserved in individuals with cerebellar degeneration when the movements are directly cued. J Cogn Neurosci. 2009;21(7):1302–10.
Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80(4):1911–31.
Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog Neurobiol. 2004;72(1):27–53.
Chen-Harris H et al. Adaptive control of saccades via internal feedback. J Neurosci. 2008;28(11):2804–13.
Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci. 2010;30(10):3715–27.
Huang YZ, Rothwell JC. The effect of short-duration bursts of high-frequency, low-intensity transcranial magnetic stimulation on the human motor cortex. Clin Neurophysiol. 2004;115(5):1069–75.
Jenkinson N, Miall RC. Disruption of saccadic adaptation with repetitive transcranial magnetic stimulation of the posterior cerebellum in humans. Cerebellum. 2010;9(4):548–55.
Robinson FR, Fuchs AF, Noto CT. Cerebellar influences on saccade plasticity. Ann NY Acad Sci. 2002;956:155–63.
Huang YZ et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–6.
Di Lazzaro V et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565(Pt 3):945–50.
Mochizuki H. The role of dorsal premotor area in reaction task: comparing the “virtual lesion” effect of paired pulse or theta burst transcranial magnetic stimulation. Exp Brain Res. 2005;167(3):414–21.
Nowak DA et al. High-frequency repetitive transcranial magnetic stimulation over the hand area of the primary motor cortex disturbs predictive grip force scaling. Eur J Neurosci. 2005;22(9):2392–6.
Huang YZ et al. The effect of continuous theta burst stimulation over premotor cortex on circuits in primary motor cortex and spinal cord. Clin Neurophysiol. 2009;120(4):796–801.
Koch G et al. Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin Neurophysiol. 2008;119(11):2559–69.
Colnaghi S et al. Multiple memory-guided saccades: movement memory improves the accuracy of memory-guided saccades. Prog Brain Res. 2008;171:425–7.
Optican LM, Robinson DA. Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol. 1980;44:1058–76.
Straube A et al. Cerebellar lesions impair rapid saccade amplitude adaptation. Neurology. 2001;57(11):2105–8.
Takeichi N, Kanek CR, Fuchs AF. Activity changes in monkey superior colliculus during saccade adaptation. J Neurophysiol. 2007;97(6):4096–107.
Leigh RJ, Zee DS. The neurology of eye movements. 4th ed. New York: Oxford University Press; 2006.
Bridgeman B. A review of the role of efference copy in sensory and oculomotor control systems. Ann Biomed Eng. 1995;23(4):409–22.
Schmahmann JD et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–60.
Fujikado T, Noda H. Saccadic eye movements evoked by microstimulation of lobule VII of the cerebellar vermis of macaque monkeys. J Physiol. 1987;394:573–94.
Noda H, Fujikado T. Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J Neurophysiol. 1987;58(2):359–78.
Noda H, Fujikado T. Involvement of Purkinje cells in evoking saccadic eye movements by microstimulation of the posterior cerebellar vermis of monkeys. J Neurophysiol. 1987;57(5):1247–61.
Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993;70(5):1741–58.
Hashimoto M, Ohtsuka K. Transcranial magnetic stimulation over the posterior cerebellum during visually guided saccades in man. Brain. 1995;118(Pt 5):1185–93.
Helmchen C, Straube A, Büttner U. Saccadic lateropulsion in Wallenberg's syndrome may be caused by a functional lesion of the fastigial nucleus. J Neurol. 1994;241(7):421–6.
Tilikete C, Koene A, Nighoghossian N, Vighetto A, Pélisson D. Saccadic lateropulsion in Wallenberg syndrome: a window to access cerebellar control of saccades? Exp Brain Res. 2006;174(3):555–65.
Waespe W, Baumgartner R. Enduring dysmetria and impaired gain adaptivity of saccadic eye movements in Wallenberg's lateral medullary syndrome. Brain. 1992;115(Pt 4):1123–46.
Epstein CM et al. Localizing the site of magnetic brain stimulation in humans. Neurology. 1990;40(4):666–70.
Rudiak D, Marg E. Finding the depth of magnetic brain stimulation: a re-evaluation. Electroencephalogr Clin Neurophysiol. 1994;93(5):358–71.
Viviani P, Velay J-L. Spatial coding of voluntary saccades. In: O'Reagan JK, Lévy-Schoen A, editors. Eye movements: from physiology to cognition. Amsterdam: Elsevier; 1987. p. 69–78.
Beauvillain C, Vergilino-Perez D, Dükic T. Spatial object representation andits use in planning eye movements. Exp Brain Res. 2005;165(3):315–27.
Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29(1):58–64.
Acknowledgments
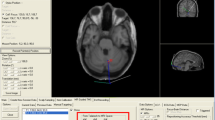
We thank Dr. Matteo Feurra for his help with the neuronavigator, and Prof Stefano Bastianello for his help in the measurement of the dorsal vermis–skull distance.
Conflict of interests
None of the authors have any financial or personal relationships that might bias the work published in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colnaghi, S., Ramat, S., D’Angelo, E. et al. Theta-Burst Stimulation of the Cerebellum Interferes with Internal Representations of Sensory-Motor Information Related to Eye Movements in Humans. Cerebellum 10, 711–719 (2011). https://doi.org/10.1007/s12311-011-0282-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-011-0282-1