Abstract
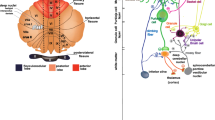
The postnatal development of the cerebellum is accomplished via a series of cytogenetic and morphogenetic events encoded in the genome. To decipher the underlying genetic basis of these events we have systematized the spatio-temporal gene expression profiles during mouse cerebellar development in the Cerebellar Development Transcriptome Database (CDT-DB). Using the CDT-DB, Ca2+-dependent activator protein for secretion 2 (CAPS2 or CADPS2) was identified as a developmentally regulated gene that is predominantly expressed in cerebellar granule cells (GCs) with an expression peak around the first or second postnatal week. CAPS2 protein is concentrated in parallel fiber (PF) terminals and is associated with secretory vesicles containing brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3). CAPS2 enhances release of BDNF and NT-3, both of which are essential for normal cerebellar development. CAPS2-deficient (CAPS2−/−) mice show reduced secretion of BDNF and NT-3; consequently, the cerebella of these mice exhibit developmental deficits, such as delayed development and increased cell death in GCs, fewer branched dendrites on Purkinje cells (PCs), and loss of the intercrural fissure. The PF-PC synapses have aberrant cytoarchitectures and electrophysiological properties. These abnormal cellular and morphological phenotypes are more severe around the cerebellar vermis, in which hypoplasia has been reported in autism patients. Moreover, CAPS2−/− mice had fewer cortical and hippocampal parvalbumin-positive interneurons and some autistic-like behavioral phenotypes. In the CAPS2 genes of some autistic patients an aberrant splicing variant and non-synonymous SNPs have been identified. These recent studies implicate CAPS2 in autism susceptibility. Therefore, CAPS2−/− mice will be a useful model animal in which to study aspects of the neuropathology and behaviors characteristic of developmental disorders.



Similar content being viewed by others
References
Sato A, Sekine Y, Saruta C, Nishibe H, Morita N, Sato Y, Sadakata T, Shinoda Y, Kojima T, Furuichi T (2008) Cerebellar development transcriptome database (CDT-DB): profiling of spatio-temporal gene expression during the postnatal development of mouse cerebellum. Neural Netw 21:1056–1069
Sadakata T, Furuichi T (2006) Identification and mRNA expression of Ogdh, QP-C, and two predicted genes in the postnatal mouse brain. Neurosci Lett 405:217–222
Sadakata T, Mizoguchi A, Sato Y, Katoh-Semba R, Fukuda M, Mikoshiba K, Furuichi T (2004) The secretory granule-associated protein CAPS2 regulates neurotrophin release and cell survival. J Neurosci 24:43–52
Sadakata T, Washida M, Furuichi T (2007) Alternative splicing variations in mouse CAPS2: differential expression and functional properties of splicing variants. BMC Neurosci 8:25
Sadakata T, Kakegawa W, Mizoguchi A, Washida M, Katoh-Semba R, Shutoh F, Okamoto T, Nakashima H, Kimura K, Tanaka M, Sekine Y, Itohara S, Yuzaki M, Nagao S, Furuichi T (2007) Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci 27:2472–2482
Sadakata T, Washida M, Iwayama Y, Shoji S, Sato Y, Ohkura T, Katoh-Semba R, Nakajima M, Sekine Y, Tanaka M, Nakamura K, Iwata Y, Tsuchiya KJ, Mori N, Detera-Wadleigh SD, Ichikawa H, Itohara S, Yoshikawa T, Furuichi T (2007) Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J Clin Invest 117:931–943
Sadakata T, Itakura M, Kozaki S, Sekine Y, Takahashi M, Furuichi T (2006) Differential distributions of the Ca2+-dependent activator protein for secretion family proteins (CAPS2 and CAPS1) in the mouse brain. J Comp Neurol 495:735–753
Shiraishi-Yamaguchi Y, Furuichi T (2007) The Homer family proteins. Genome Biol 8:206
Shiraishi Y, Mizutani A, Bito H, Fujisawa K, Narumiya S, Mikoshiba K, Furuichi T (1999) Cupidin, an isoform of Homer/Vesl, interacts with the actin cytoskeleton and activated rho family small GTPases and is expressed in developing mouse cerebellar granule cells. J Neurosci 19:8389–8400
Huang J, Sakai R, Furuichi T (2006) The docking protein Cas links tyrosine phosphorylation signaling to elongation of cerebellar granule cell axons. Mol Biol Cell 17:3187–3196
Huang J, Furuya A, Furuichi T (2007) Very-KIND, a KIND domain containing RasGEF, controls dendrite growth by linking Ras small GTPases and MAP2. J Cell Biol 179:539–552
Yoshikawa F, Sato Y, Tohyama K, Akagi T, Hashikawa T, Nagakura-Takagi Y, Sekine Y, Morita N, Baba H, Suzuki Y, Sugano S, Sato A, Furuichi T (2008) Opalin, a transmembrane sialylglycoprotein located in the central nervous system myelin paranodal loop membrane. J Biol Chem 283:20830–20840
Aruga J, Yoshikawa F, Nozaki Y, Sakaki Y, Toyoda A, Furuichi T (2007) An oligodendrocyte enhancer in a phylogenetically conserved intron region of the mammalian myelin gene Opalin. J Neurochem 102:1533–1547
Speidel D, Varoqueaux F, Enk C, Nojiri M, Grishanin RN, Martin TF, Hofmann K, Brose N, Reim K (2003) A family of Ca2+-dependent activator proteins for secretion: comparative analysis of structure, expression, localization, and function. J Biol Chem 278:52802–52809
Cisternas FA, Vincent JB, Scherer SW, Ray PN (2003) Cloning and characterization of human CADPS and CADPS2, new members of the Ca2+-dependent activator for secretion protein family. Genomics 81:279–291
Berwin B, Floor E, Martin TF (1998) CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron 21:137–145
Tandon A, Bannykh S, Kowalchyk JA, Banerjee A, Martin TF, Balch WE (1998) Differential regulation of exocytosis by calcium and CAPS in semi-intact synaptosomes. Neuron 21:147–154
Renden R, Berwin B, Davis W, Ann K, Chin CT, Kreber R, Ganetzky B, Martin TF, Broadie K (2001) Drosophila CAPS is an essential gene that regulates dense-core vesicle release and synaptic vesicle fusion. Neuron 31:421–437
Ann K, Kowalchyk JA, Loyet KM, Martin TF (1997) Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J Biol Chem 272:19637–19640
Elhamdani A, Martin TF, Kowalchyk JA, Artalejo CR (1999) Ca2+-dependent activator protein for secretion is critical for the fusion of dense-core vesicles with the membrane in calf adrenal chromaffin cells. J Neurosci 19:7375–7383
Waselle L, Gerona RR, Vitale N, Martin TF, Bader MF, Regazzi R (2005) Role of phosphoinositide signaling in the control of insulin exocytosis. Mol Endocrinol 19:3097–3106
Speidel D, Salehi A, Obermueller S, Lundquist I, Brose N, Renstrom E, Rorsman P (2008) CAPS1 and CAPS2 regulate stability and recruitment of insulin granules in mouse pancreatic beta cells. Cell Metab 7:57–67
Rybkin II, Kim MS, Bezprozvannaya S, Qi X, Richardson JA, Plato CF, Hill JA, Bassel-Duby R, Olson EN (2007) Regulation of atrial natriuretic peptide secretion by a novel Ras-like protein. J Cell Biol 179:527–537
Eisenhofer G, Huynh TT, Elkahloun A, Morris JC, Bratslavsky G, Linehan WM, Zhuang Z, Balgley BM, Lee CS, Mannelli M, Lenders JW, Bornstein SR, Pacak K (2008) Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. Am J Physiol Endocrinol Metab 295:E1223–1233
Grishanin RN, Klenchin VA, Loyet KM, Kowalchyk JA, Ann K, Martin TF (2002) Membrane association domains in Ca2+-dependent activator protein for secretion mediate plasma membrane and dense-core vesicle binding required for Ca2+-dependent exocytosis. J Biol Chem 277:22025–22034
Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF (2004) CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron 43:551–562
Osborne SL, Wallis TP, Jimenez JL, Gorman JJ, Meunier FA (2007) Identification of secretory granule phosphatidylinositol 4,5-bisphosphate-interacting proteins using an affinity pulldown strategy. Mol Cell Proteomics 6:1158–1169
James DJ, Khodthong C, Kowalchyk JA, Martin TF (2008) Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol 182:355–366
Speese S, Petrie M, Schuske K, Ailion M, Ann K, Iwasaki K, Jorgensen EM, Martin TF (2007) UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J Neurosci 27:6150–6162
Hammarlund M, Watanabe S, Schuske K, Jorgensen EM (2008) CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. J Cell Biol 180:483–491
Speidel D, Bruederle CE, Enk C, Voets T, Varoqueaux F, Reim K, Becherer U, Fornai F, Ruggieri S, Holighaus Y, Weihe E, Bruns D, Brose N, Rettig J (2005) CAPS1 regulates catecholamine loading of large dense-core vesicles. Neuron 46:75–88
Brunk I, Blex C, Speidel D, Brose N, Ahnert-Hilger G (2008) Ca2+-dependent activator proteins of secretion promote vesicular monoamine uptake. J Biol Chem 284:1050–1056
Jockusch WJ, Speidel D, Sigler A, Sorensen JB, Varoqueaux F, Rhee JS, Brose N (2007) CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell 131:796–808
Fujita Y, Xu A, Xie L, Arunachalam L, Chou TC, Jiang T, Chiew SK, Kourtesis J, Wang L, Gaisano HY, Sugita S (2007) Ca2+-dependent activator protein for secretion 1 is critical for constitutive and regulated exocytosis but not for loading of transmitters into dense core vesicles. J Biol Chem 282:21392–21403
Zhou KM, Dong YM, Ge Q, Zhu D, Zhou W, Lin XG, Liang T, Wu ZX, Xu T (2007) PKA activation bypasses the requirement for UNC-31 in the docking of dense core vesicles from C. elegans neurons. Neuron 56:657–669
Cai T, Fukushige T, Notkins AL, Krause M (2004) Insulinoma-Associated Protein IA-2, a Vesicle Transmembrane Protein, Genetically Interacts with UNC-31/CAPS and Affects Neurosecretion in Caenorhabditis elegans. J Neurosci 24:3115–3124
Gracheva EO, Burdina AO, Touroutine D, Berthelot-Grosjean M, Parekh H, Richmond JE (2007) Tomosyn negatively regulates CAPS-dependent peptide release at Caenorhabditis elegans synapses. J Neurosci 27:10176–10184
Binda AV, Kabbani N, Levenson R (2005) Regulation of dense core vesicle release from PC12 cells by interaction between the D2 dopamine receptor and calcium-dependent activator protein for secretion (CAPS). Biochem Pharmacol 69:1451–1461
Charlie NK, Schade MA, Thomure AM, Miller KG (2006) Presynaptic UNC-31 (CAPS) is required to activate the Gαs pathway of the Caenorhabditis elegans synaptic signaling network. Genetics 172:943–961
Austin MC, Schultzberg M, Abbott LC, Montpied P, Evers JR, Paul SM, Crawley JN (1992) Expression of tyrosine hydroxylase in cerebellar Purkinje neurons of the mutant tottering and leaner mouse. Brain Res Mol Brain Res 15:227–240
Takada M, Sugimoto T, Hattori T (1993) Tyrosine hydroxylase immunoreactivity in cerebellar Purkinje cells of the rat. Neurosci Lett 150:61–64
Verney C, Grzanna R, Farkas E (1982) Distribution of dopamine-β-hydroxylase-like immunoreactive fibers in the rat cerebellar cortex during ontogeny. Dev Neurosci 5:369–374
Fernandes ML, Saad MJ, Velloso LA (2001) Effects of age on elements of insulin-signaling pathway in central nervous system of rats. Endocrine 16:227–234
de LAFML, Saad MJ, Velloso LA (1999) Insulin induces tyrosine phosphorylation of the insulin receptor and SHC, and SHC/GRB2 association in cerebellum but not in forebrain cortex of rats. Brain Res 826:74–82
Sherrard RM, Bower AJ (2002) Climbing fiber development: do neurotrophins have a part to play? Cerebellum 1:265–275
Katoh-Semba R, Takeuchi IK, Semba R, Kato K (1997) Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem 69:34–42
Katoh-Semba R, Takeuchi IK, Semba R, Kato K (2000) Neurotrophin-3 controls proliferation of granular precursors as well as survival of mature granule neurons in the developing rat cerebellum. J Neurochem 74:1923–1930
Das KP, Chao SL, White LD, Haines WT, Harry GJ, Tilson HA, Barone S Jr (2001) Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience 103:739–761
Lindholm D, Hamner S, Zirrgiebel U (1997) Neurotrophins and cerebellar development. Perspect Dev Neurobiol 5:83–94
Lindholm D, Castren E, Tsoulfas P, Kolbeck R, Berzaghi Mda P, Leingartner A, Heisenberg CP, Tessarollo L, Parada LF, Thoenen H (1993) Neurotrophin-3 induced by tri-iodothyronine in cerebellar granule cells promotes Purkinje cell differentiation. J Cell Biol 122:443–450
Segal RA, Pomeroy SL, Stiles CD (1995) Axonal growth and fasciculation linked to differential expression of BDNF and NT3 receptors in developing cerebellar granule cells. J Neurosci 15:4970–4981
Gao WQ, Zheng JL, Karihaloo M (1995) Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) act at later stages of cerebellar granule cell differentiation. J Neurosci 15:2656–2667
Larkfors L, Lindsay RM, Alderson RF (1996) Characterization of the responses of Purkinje cells to neurotrophin treatment. J Neurochem 66:1362–1373
Doughty ML, Lohof A, Campana A, Delhaye-Bouchaud N, Mariani J (1998) Neurotrophin-3 promotes cerebellar granule cell exit from the EGL. Eur J Neurosci 10:3007–3011
Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, Tolias KF, Bikoff JB, Hong EJ, Greenberg ME, Segal RA (2007) Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron 55:53–68
Jones KR, Farinas I, Backus C, Reichardt LF (1994) Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76:989–999
Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA (1997) Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron 19:269–281
Carter AR, Chen C, Schwartz PM, Segal RA (2002) Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J Neurosci 22:1316–1327
Bates B, Rios M, Trumpp A, Chen C, Fan G, Bishop JM, Jaenisch R (1999) Neurotrophin-3 is required for proper cerebellar development. Nat Neurosci 2:115–117
Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL (1988) Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med 318:1349–1354
Pierce K, Courchesne E (2001) Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry 49:655–664
Martinez A, Alcantara S, Borrell V, Del Rio JA, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silos-Santiago I, Soriano E (1998) TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J Neurosci 18:7336–7350
Ernfors P, Lee KF, Kucera J, Jaenisch R (1994) Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell 77:503–512
World Health Organization (1992) The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. WHO, Geneva
American Psychiatric Association., American Psychiatric Association. Task Force on DSM-IV (1994) Diagnostic and statistical manual of mental disorders: DSM-IV, 4th edn. American Psychiatric Association, Washington, DC
Muhle R, Trentacoste SV, Rapin I (2004) The genetics of autism. Pediatrics 113:e472–486
Folstein SE, Rosen-Sheidley B (2001) Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet 2:943–955
Abrahams BS, Geschwind DH (2008) Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 9:341–355
IMGSAC (2001) Further characterization of the autism susceptibility locus AUTS1 on chromosome 7q. Hum Mol Genet 10:973–982
Bauman M, Kemper TL (1985) Histoanatomic observations of the brain in early infantile autism. Neurology 35:866–874
Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ (2008) Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum 7:406–416
Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J (2007) Mapping early brain development in autism. Neuron 56:399–413
Takarae Y, Minshew NJ, Luna B, Krisky CM, Sweeney JA (2004) Pursuit eye movement deficits in autism. Brain 127:2584–2594
Manjiviona J, Prior M (1995) Comparison of Asperger syndrome and high-functioning autistic children on a test of motor impairment. J Autism Dev Disord 25:23–39
Casanova MF, Buxhoeveden D, Gomez J (2003) Disruption in the inhibitory architecture of the cell minicolumn: implications for autisim. Neuroscientist 9:496–507
Aman MG (2004) Management of hyperactivity and other acting-out problems in patients with autism spectrum disorder. Semin Pediatr Neurol 11:225–228
Aman MG, Langworthy KS (2000) Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J Autism Dev Disord 30:451–459
Richdale AL, Prior MR (1995) The sleep/wake rhythm in children with autism. Eur Child Adolesc Psychiatry 4:175–186
Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH Jr., Dawson G, Gordon B, Gravel JS, Johnson CP, Kallen RJ, Levy SE, Minshew NJ, Ozonoff S, Prizant BM, Rapin I, Rogers SJ, Stone WL, Teplin SW, Tuchman RF, Volkmar FR (2000) Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology 55:468–479
Deacon SW, Serpinskaya AS, Vaughan PS, Lopez Fanarraga M, Vernos I, Vaughan KT, Gelfand VI (2003) Dynactin is required for bidirectional organelle transport. J Cell Biol 160:297–301
Waterman-Storer CM, Karki SB, Kuznetsov SA, Tabb JS, Weiss DG, Langford GM, Holzbaur EL (1997) The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc Natl Acad Sci U S A 94:12180–12185
Acknowledgments
This study was supported by grants-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT), the Japan Science and Technology Agency (JST), the Japan Society for the Promotion of Science (JSPS), and the Institute of Physical and Chemical Research (RIKEN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadakata, T., Furuichi, T. Developmentally Regulated Ca2+-Dependent Activator Protein for Secretion 2 (CAPS2) is Involved in BDNF Secretion and is Associated with Autism Susceptibility. Cerebellum 8, 312–322 (2009). https://doi.org/10.1007/s12311-009-0097-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-009-0097-5