Abstract
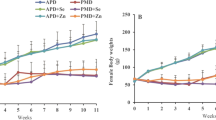
The study was designed to investigate the effects of dietary zinc deficiency and supplementation on antioxidant system viz. superoxide-dismutase, glutathione reductase, glutathione peroxidase, glutathione- S-transferase, catalase and sulfhydryls levels (GSH, TSH, NPSH and PBSH) in testes of Wistar rats. Pre-pubertal rats were divided into two groups with 6 sub-groups each viz. zinc control (ZC), pair fed (PF), zinc deficient (ZD), zinc control supplementation (ZCS), pair-fed supplementation (PFS) and zinc deficient supplementation (ZDS). Experiments were set for 2- and 4-weeks followed by 4 weeks of zinc supplementation. The zinc deficient group animals exhibited significant decrease in gonado-somatic index (2- and 4- weeks), sulfhydryls levels, GSH, GPx, GR (2 and 4-weeks) and GST concentration (2-weeks). However, after zinc supplementation significant improvement in gonadosomatic index, SH, GSH, antioxidant enzyme levels (GR, GPx, and GST) in deficient groups has been observed. Zinc deficiency during pre-pubertal period affected growth and caused dysregulation of the glutathione antioxidant system. The significant alterations in the levels of antioxidant enzymes and non-enzymatic antioxidant system (GSH and SH) in zinc deficient groups could be due to alleviated generation of free radicals, causative factor for increased oxidative stress which may lead to infertility as oxidative stress is a common pathology seen during infertility. Altered antioxidant system and sulfhydryls levels in testes due to dietary zinc deficiency reflect the significance of optimum zinc for maintaining homeostatic balance in gonadal physiology. Supplementing zinc for 4 weeks could reduce the redox imbalance which may help in alleviating oxidative stress induced alterations in testes.
Similar content being viewed by others
References
Krezel A, Maret W. The biological inorganic chemistry of zinc ions. Arch Biochem Biophys. 2016;611:3–19.
Prasad AS, Bao B. Molecular mechanisms of zinc as a pro-antioxidant mediator: clinical therapeutic implications. Antioxidants (Basel). 2019;8(6):164. https://doi.org/10.3390/antiox8060164.
Vickram S, Rohini K, Srinivasan S, Veenakumari DN, Archana K, Anbarasu K, et al. Role of zinc (Zn) in human reproduction: a journey from initial spermatogenesis to childbirth. Int J Mol Sci. 2021;22:2188–205.
Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem Rev. 2009;109:4682–707.
Oteiza PI. Zinc and the modulation of redox homeostasis. Free Radic Biol Med. 2012;12(53):1748–59.
Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc: zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25(1):11–24.
Kambe T, Taylor KM, Dax Fu. Zinc transporters and their functional integration in mammalian cells. J Biol Chem. 2021. https://doi.org/10.1016/j.jbc.2021.100320.
Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE. 2012;7(11): e50568. https://doi.org/10.1371/journal.pone.0050568.
Akhtar S. Zinc status in South Asian populations–an update. J Health Popul Nutr. 2013;31(2):139–49.
Gupta S, Brazier AKM. Zinc deficiency in low- and middle-income countries: prevalence and approaches for mitigation. J Hum Nutr Diet. 2020;33:624–43.
Björndahl L, Kvist U. A model for the importance of zinc in the dynamics of human sperm chromatin stabilization after ejaculation in relation to sperm DNA vulnerability. Syst Biol Reprod Med. 2011;57(1–2):86–92.
Roy B, Baghel RPS, Mohanty TK, Mondal G. Zinc and male reproduction in domestic animals: a review. Indian J Anim Nutr. 2013;30(4):339–50.
Foresta C, Garolla A, Cosci I, Menegazzo M, Ferigo M, Gandin V, et al. Role of zinc trafficking in male fertility: from germ to sperm. Hum Reprod. 2014;29(6):1134–45.
Fallah A, Mohammad-Hasani A, Colagar AH. Zinc is an essential element for male fertility: a review of Zn roles in men’s health, germination, sperm quality, and fertilization. J Reprod Infertil. 2018;19:069–80.
Zigo M, Kerns K, Sen S, Essien C, Oko R, Xu D, et al. Zinc is a master-regulator of sperm function associated with binding, motility, and metabolic modulation during porcine sperm capacitation. Commun Biol. 2022;5:538. https://doi.org/10.1038/s42003-022-03485-8.
Merrells KJ, Blewett H, Jaamieson JA, Taylor CG, Suh M. Relationship between abnormal sperm physiology induced by dietary zinc deficiency and lipid composition in testes of growing rats. Br J Nutr. 2009;102:226–32.
Croxford TP, McCormick NH, Kelleher SL. Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J Nutr. 2011;141(3):359–65.
Kumari D, Nair N, Bedwal RS. Effect of dietary zinc deficiency on testes of Wistar rats: morphometric and cell quantification studies. J Trace Elem Med Biol. 2011;25:47–53.
Galano A, Alvarez-Idaboy JR. Glutathione: mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 2011;1:1763–71.
Jozefczak M, Remans T, Vangronsveld J, Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci. 2012;13:3145–75.
Aquilano K, Baldelli S, Ciriolo MR. Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol. 2014;5:196. https://doi.org/10.3389/fphar.2014.00196.
Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol. 2005;5:5–17.
Adeoye O, Olawumi J, Opeyemi A, Christiania O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist Reprod. 2018;22:61–6.
Couto N, Wood J, Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med. 2016;95:27–42.
Ighodaro M, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med. 2018;54:287–93.
Tan BL, Norhaizan ME, Liew WP, Rahman HS. Antioxidant and oxidative stress: a mutual interplay inage-related diseases. Front Pharmacol. 2018;9:1162. https://doi.org/10.3389/fphar.2018.01162.
Fukai T, Fukai MU. Superoxide dismutases: role in redox signaling, vascular function and diseases. Antioxid Redox Signal. 2011;15(6):1583–606.
Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1(1):15–24.
Zhou Z, Kang YJ. Cellular and subcellular localization of catalase in the heart of transgenic mice. J Histochem Cytochem. 2000;48(5):585–94.
Kaneko T, Iuchi Y, Kobayashi T, Fujii T, Saito H, Kurachi H, et al. The expression of glutathione reductase in the male reproductive system of rats supports the enzymatic basis of glutathione function in spermatogenesis. Eur J Biochem. 2002;269(5):1570–8.
Palamanda JR, Kehrer J. Inhibition of protein carbonyl formation and lipid peroxidation by glutathione in rat liver microsomes. Arch Biochem Biophys. 1992;293(1):103–9.
Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–69.
Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–90.
Warholm M, Guthenberg C, Von Bohr C, Mannervik B. Glutathione transferases from human liver. Methods Enzymol. 1985;113:499–504.
Sedlak J, Lindsay RH. Estimation of total protein bound and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94.
Marklund S, Marklund G. Involvement of superoxide anion radical in the autooxidation of pyrogallol and convenient assay of superoxide dismutase. Eur J Biochem. 1974;47:467–74.
Geller BL, Winge DR. A method for distinguishing Cu, Zn and Mn-containing superoxide dismutases. Anal Biochem. 1983;128(1):86–92.
Zhu X, Yu C, Wu W, Shi L, Jiang C, Wang L, et al. Zinc transporter ZIP12 maintains zinc homeostasis and protects spermatogonia from oxidative stress during spermatogenesis. Reprod Biol Endocrinol. 2022;20(1):17. https://doi.org/10.1186/s12958-022-00893-7.
Harchegani AB, Dahan H, Tahmasbpour E, Kaboutaraki HB, Shahriary A. Effects of zinc deficiency on impaired spermatogenesis and male infertility: the role of oxidative stress, inflammation and apoptosis. Hum Fertil. 2018. https://doi.org/10.1080/14647273.2018.1494390.
Gul M, Kutay FZ, Temocin S, Hanninen O. Cellular and clinical implications of glutathione. Indian J Exp Biol. 200l; 38(7):625–34.
Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214.
Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Asp Med. 2009;30:1–12.
El-Seweidy MM, Hashem RM, Abo-El-matty DM, Mohamed RH. Frequent inadequate supply of micronutrients in fast food induces oxidative stress and inflammation in testicular tissues of weanling rats. J Pharm Pharmacol. 2008;60(9):1237–42.
Ebuchi OAT, Akande GA. Effect of zinc deficiency on memory, oxidative stress and blood chemistry in rats. J Adv Med Dent Sci Res. 2009;2(3):74–82.
Crisol L, Matorras R, Aspichueta F, Expósito A, Hernández ML, Ruiz-Larrea MB, et al. Glutathione peroxidase activity in seminal plasma and its relationship to classical sperm parameters and in vitro fertilization-intracytoplasmic sperm injection outcome. Fertil Steril. 2012;97:852–7.
Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI. Dietary zinc induced changes in the activity of enzymes and the levels of free radicals lipids and protein electrophoretic behaviour in growing rats. Toxicology. 2002;175:223–34.
Ebisch IMW, Thomas CMG, Peters WHM, Bract DDM, Steegers-Theunissen RPM. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of sub-fertility. Hum Reprod Update. 2007;13(2):163–74.
Fujii J, Iuchi Y, Matsuki S, Ishii T. Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J Androl. 2003;5:231–42.
Trist B, Hilton JB, Crouch PJ, Hare DJ, Double KL. Superoxide dismutase 1 in health and disease: how a frontline antioxidant becomes neurotoxic. Angew Chem Int Ed Engl. 2021;60(17):9215–46.
Gu W, Morales C, Hecht NB. In male mouse germ cells, copper-zinc superoxide dismutase utilizes alternative promoters that produce multiple transcripts with different translation potential. J Biol Chem. 1995;270(1):236–43.
Oteiza PI, Olin KL, Fraga CG, Keen CL. Oxidant defense systems in testes from zinc deficient rats. Proc Soc Exp Biol Med. 1996;213:85–91.
Geraghty AC, Kaufer D. Glucocorticoid regulation of reproduction. Adv Exp Med Biol. 2015;872:253–78.
Whirledge S, Cidlowski JA. Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metab. 2017;28(6):399–415.
Bauché F, Fouchard MH, Jégou B. Antioxidant system in rat testicular cells. FEBS Lett. 1994;349:392–6.
Omu AE, Al-Azemi MK, Kehinde EO, Anim JT, Oriowo MA, Mathew TC. Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med Princ Pract. 2008;17:108–16.
Mousavi Esfiokhi SH, Norouzian MA, Najafi A. Effect of different sources of dietary zinc on sperm quality and oxidative parameters. Front Vet Sci. 2023;10:1134244. https://doi.org/10.3389/fvets.2023.1134244.
Kumari D, Nair N, Bedwal RS. Testicular apoptosis after dietary zinc deficiency: ultrastructural and TUNEL studies. Syst Biol Reprod Med. 2011;57:233–43.
Allouche-Fitoussi D, Breitbart H. The role of zinc in male fertility. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21207796.
Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J Clin Diagn Res. 2017;11(5):IE01–5.
Dutta S, Sengupta P, Slama P, Roychoudhury S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int J Mol Sci. 2021;22(18):10043.
Huang C, Cao X, Pang D, Li C, Luo Q, Zou Y, et al. Is male infertility associated with increased oxidative stress in seminal plasma? A-meta analysis. Oncotarget. 2018; 11;9(36):24494–24513. https://doi.org/10.18632/oncotarget.25075
Mannucci A, Argento FR, Fini E, Coccia ME, Taddei N, Becatti M, et al. The impact of oxidative stress in male infertility. Front Mol Biosci. 2022;8: 799294. https://doi.org/10.3389/fmolb.2021.799294.
Aitken RJ, Drevet JR, Moazamian A, Gharagozloo P. Male infertility and oxidative stress: a focus on the underlying mechanisms. Antioxidants. 2022;11:306. https://doi.org/10.3390/antiox11020306.
Acknowledgements
Financial support for the conduct of the research provided to Dr. Deepa Kumari-Post Doctoral Fellowship by University Grants Commission (UGC, New Delhi), vide letter No.- F.15- 1/2011-12/PDFWM-2011-12-GE-RAJ-3826 [SA-II] is acknowledged. The authors gratefully acknowledge Department of Zoology, Centre for Advanced Studies, University of Rajasthan, Jaipur, India for providing the necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, D., Nair, N. & Bedwal, R.S. Effects of Dietary Zinc Deficiency and Supplementation on Prepubertal Rat Testes: Sulfhydryl and Antioxidant Status. Ind J Clin Biochem (2023). https://doi.org/10.1007/s12291-023-01167-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12291-023-01167-8