Abstract
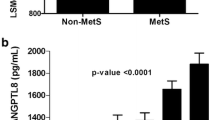
The metabolic syndrome (MetS) is a serious public health issue that affects people all over the world. Notably, insulin resistance, prothrombotic activity, and inflammatory state are associated with MetS. This study aims to explore the relationship between cytokines and tumor necrosis factor-α (TNF-α), pancreatic-derived factor (PANDER), and interleukin (IL-)-37 and the accumulation of MetS components. Eligible participants were divided into four groups as follows: group 1, patients with dyslipidemia; group 2, patients with dyslipidemia and obesity; group 3, patients with dyslipidemia, obesity, and hypertension; and group 4, patients with dyslipidemia, obesity, hypertension, and hyperglycemia. This study exhibited that serum levels of TNF-α and PANDER were significantly elevated (P < 0.001) in the MetS groups, while IL-37 level and IL-37 mRNA expression were significantly decreased (P < 0.001) relative to healthy controls. Moreover, this study has revealed significant correlations (P < 0.001) between MetS components and TNF-α, PANDER, and IL-37 levels in MetS patients. The aforementioned results suggested the association between the proinflammatory cytokine (TNF-α and PANDER) and anti-inflammatory cytokine (IL-37) with the accumulation of MetS components. Hence, the overall outcome indicated that PANDER and IL-37 may be considered novel biomarkers associated with increased risk of MetS and can be used as a promising therapeutic target in preventing, ameliorating, and treating metabolic disorders.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi:https://doi.org/10.1016/S0140-6736(05)66378-7.
Akdas S, Turan B, Durak A, Aribal Ayral P, Yazihan N. The Relationship between Metabolic Syndrome Development and Tissue Trace Elements Status and Inflammatory Markers. Biol Trace Elem Res. 2020;198(1):16–24. doi:https://doi.org/10.1007/s12011-020-02046-6.
van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Dysfunctional adipose tissue and low-grade inflammation in the management of the metabolic syndrome: current practices and future advances. F1000Res. 2016;5:F1000 Faculty Rev-2515. doi:https://doi.org/10.12688/f1000research.8971.1.
Dallmeier D, Larson MG, Vasan RS, Keaney JF Jr, Fontes JD, Meigs JB, et al. Metabolic syndrome and inflammatory biomarkers: a community-based cross-sectional study at the Framingham Heart Study. Diabetol Metab Syndr. 2012;4(1):28. doi:https://doi.org/10.1186/1758-5996-4-28.
Bălăşoiu M, Bălăşoiu AT, Stepan AE, Dinescu SN, Avramescu CS, Dumitrescu D, et al. Proatherogenic adipocytokines levels in metabolic syndrome. Rom J Morphol Embryol. 2014; 55(1):29–33. Available from: https://rjme.ro/RJME/resources/files/550114029033.pdf.
Cao X, Yang C, Lai F, Dinescu SN, Avramescu CS, Dumitrescu D, et al. Elevated circulating level of a cytokine, pancreatic-derived factor, is associated with metabolic syndrome components in a Chinese population. J Diabetes Investig. 2016;7(4):581–6. doi:https://doi.org/10.1111/jdi.12437.
Navarro-Gonzalez JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19(13):433–42. doi:https://doi.org/10.1681/ASN.2007091048.
Sharma P. Inflammation and the metabolic syndrome. Indian J Clin Biochem. 2011;26(4):317–8. doi:https://doi.org/10.1007/s12291-011-0175-6.
Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582(1):117–31. doi:https://doi.org/10.1016/j.febslet.2007.11.051.
Marques-Vidal P, Bastardot F, von Känel R, Paccaud F, Preisig M, Waeber G, et al. Association between circulating cytokine levels, diabetes and insulin resistance in a population-based sample (CoLaus study). Clin Endocrinol (Oxf). 2013;78(2):232–41. doi:https://doi.org/10.1111/j.1365-2265.2012.04384.x.
Cao X, Gao Z, Robert CE, Greene S, Xu G, Xu W, et al. Pancreatic-Derived Factor (FAM3B), a Novel Islet Cytokine, Induces Apoptosis of Insulin-Secreting β-Cells. Diabetes. 2003;52(9):2296–303. doi:https://doi.org/10.2337/diabetes.52.9.2296.
Zhuang X, Wu B, Li J, Shi H, Jin B, Luo X. The emerging role of interleukin-37 in cardiovascular diseases. Immun Inflam Dis. 2017;5(3):373–9. doi:https://doi.org/10.1002/iid3.159.
Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ, et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5(1):4711. doi:https://doi.org/10.1038/ncomms5711.
Parikh RM, Mohan V. Changing definitions of metabolic syndrome. Indian J Endocrinol Metab. 2012;16(1):7–12. doi:https://doi.org/10.4103/2230-8210.91175.
Abdel-Moneim A, Mahmoud B, Sultan EA, Mahmoud R. Relationship of leukocytes, platelet indices and adipocytokines in metabolic syndrome patients. Diabetes Metab Syndr. 2019;13(1):874–80. doi:https://doi.org/10.1016/j.dsx.2018.12.016.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. https://doi.org/10.1093/clinchem/18.6.499.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. doi:https://doi.org/10.2337/diacare.27.6.1487.
Musialik K. The influence of chosen adipocytokines on blood pressure values in patients with metabolic syndrome. Kardiol Pol. 2012;70(12):1237–42.
Mohammadi M, Gozashti MH, Aghadavood M, Mehdizadeh MR, Hayatbakhsh MM. Clinical Significance of Serum IL-6 and TNF-α Levels in Patients with Metabolic Syndrome. Rep Biochem Mol Biol. 2017; 6 (1): 74 – 9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5643447/pdf/rbmb-6-074.pdf.
van -Harmelen V, Dicker A, Rydén M, Hauner H, Lönnqvist F, Näslund E, et al. Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes. 2002;51(7):2029–36. doi:https://doi.org/10.2337/diabetes.51.7.2029.
Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62(11):1543–52. doi:https://doi.org/10.1016/j.metabol.2013.07.001.
Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010;11(1):145–56. doi:https://doi.org/10.1159/000289203.
Swaroop JJ, Rajarajeswari D, Naidu JN. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2012;135:127–30. doi:https://doi.org/10.4103/0971-5916.93435.
Buemi M, Marino D, Floccari F, Ruello A, Nostro L, Aloisi C, et al. Effect of interleukin-8 and ICAM-1 on calcium-dependent outflow of K+ in erythrocytes from subjects with essential hypertension. Curr Med Res Opin. 2004;20(1):19–24. doi:https://doi.org/10.1185/030079903125002720.
Wang C, Guan Y, Yang J. Cytokines in the Progression of Pancreatic β-Cell Dysfunction. Int J Endocrinol. 2010; 2010:515136. doi: https://doi.org/10.1155/2010/515136.
Robert-Cooperman CE, Carnegie JR, Wilson CG, Yang J, Cook JR, Wu J, et al. Targeted disruption of pancreatic-derived factor (PANDER, FAM3B) impairs pancreatic beta-cell function. Diabetes. 2010;59(9):2209–18. doi:https://doi.org/10.2337/db09-1552.
Wang C, Burkhardt BR, Guan Y, Yang J. Role of pancreatic-derived factor in type 2 diabetes: evidence from pancreatic β cells and liver. Nutr Rev. 2012;70(2):100–6. doi:https://doi.org/10.1111/j.1753-4887.2011.00457.x.
MarElia CB, Kuehl MN, Shemwell TA, Almanb AC, Burkhardta BR. Circulating PANDER concentration is associated with increased HbA1c and fasting blood glucose in Type 2 diabetic subjects. J Clin Transl Endocrinol. 2018;11:26–30. doi:https://doi.org/10.1016/j.jcte.2018.02.003.
Ratliff WA, Athanason MG, Chechele AC, Melanie NK, Amanda MF, Catherine BM, et al. Hepatic nutrient and hormonal regulation of the PANcreatic-DERived factor (PANDER) promoter. Mol Cell Endocrinol. 2015;413:101–12. doi:https://doi.org/10.1016/j.mce.2015.05.040.
Wilson CG, Robert-Cooperman CE, Burkhardt BR. PA. Ncreatic-DERived factor: Novel hormone PANDERing to glucose regulation. FEBS Lett. 2011;585(14):2137–43. doi:https://doi.org/10.1016/j.febslet.2011.05.059.
Athanason MG, Ratliff WA, Chaput D, MarElia CB, Kuehl MN, Stevens SM, et al. Quantitative proteomic profiling reveals hepatic lipogenesis and liver X receptor activation in the PANDER transgenic model. Mol Cell Endocrinol. 2016;436:41–9. doi:https://doi.org/10.1016/j.mce.2016.07.009.
Graus-Nunes F, Souza-Mello V. The renin-angiotensin system as a target to solve the riddle of endocrine pancreas homeostasis. Biomed Pharmacother. 2019;109:639–45. doi:https://doi.org/10.1016/j.biopha.2018.10.191.
Li J, Chi Y, Wang C, Wu J, Yang H, Zhang D, et al. Pancreatic-derived factor promotes lipogenesis in the mouse liver: role of the Forkhead box 1 signaling pathway. Hepatology. 2011;53(6):1906–16. doi:https://doi.org/10.1002/hep.24295.
Rashid S, Watanabe T, Sakaue T, Lewis GF. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: the combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin Biochem. 2003;36(6):421–9. doi:https://doi.org/10.1016/s0009-9120(03)00078-x.
Britton KA, Fox CS. Ectopic Fat Depots and Cardiovascular Disease. Circulation. 2011;124(24):e837–e41. doi:https://doi.org/10.1161/CIRCULATIONAHA.111.077602.
Sakai NS, Taylor SA, Chouhan MD. Obesity, metabolic disease and the pancreas—Quantitative imaging of pancreatic fat. Br J Radiol. 2018;91:20180267. doi:https://doi.org/10.1259/bjr.20180267.
Koroglu N, Temel Yuksel I, Aslan Cetin B, Esra NT, Nura FT, Ugur T, et al. Increased pancreatic-derived factor (PANDER) levels in gestational diabetes mellitus. Gynecol Endocrinol. 2019;35(10):866–8. doi:https://doi.org/10.1080/09513590.2019.1599856.
Allam G, Gaber AM, Othman SI, Abdel-Moneim A. The potential role of interleukin-37 in infectious diseases. Int Rev Immunol. 2020;39(1):3–10. doi:https://doi.org/10.1080/08830185.2019.1677644.
Pei B, Xu S, Liu T, Pan F, Xu J, Ding C. Associations of the IL-1F7 gene polymorphisms with rheumatoid arthritis in Chinese Han population. Int J Immunogenet. 2013;40(3):199–203. doi:https://doi.org/10.1111/iji.12007.
Ballak DB, Stienstra R, Tack CJ, Dinarello CA, van Diepen JA. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine. 2015;75(2):280–90. doi:https://doi.org/10.1016/j.cyto.2015.05.005.
Hardie DG, Ross FA, Hawley SA. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62. doi:https://doi.org/10.1038/nrm3311.
Ceddia RB. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol Cell Endocrinol. 2013;366(2):194–203. doi:https://doi.org/10.1016/j.mce.2012.06.014.
Quirk S, Agrawal DK. Immunobiology of IL-37: mechanism of action and clinical perspectives. Expert Rev Clin Immunol. 10(12):1703–9. doi: https://doi.org/10.1586/1744666X.2014.971014.
Liu J, Lin J, He S, Wang B, Liu J, Duan Y, et al. Transgenic Overexpression of IL-37 Protects Against Atherosclerosis and Strengthens Plaque Stability. Cell Physiol Biochem. 2018;45(3):1034–50. doi:https://doi.org/10.1159/000487344.
Li T, Li H, Li W, Chen S, Feng T, Jiao W, et al. Interleukin-37 sensitize the elderly type 2 diabetic patients to insulin therapy through suppressing the gut microbiota dysbiosis. Mol Immunol. 2019;112:322–9. doi:https://doi.org/10.1016/j.molimm.2019.06.008.
Ballak DB, Li S, Cavalli G, Stahl JL, Tengesdal IW, van Diepen JA, et al. Interleukin-37 treatment of mice with metabolic syndrome improves insulin sensitivity and reduces pro-inflammatory cytokine production in adipose tissue. J Biol Chem. 2018;293(37):14224–36. doi:https://doi.org/10.1074/jbc.RA118.003698.
Lin XY, Guo XJ, He YZ, Hou SF, Zhu HB, Cheng Y, et al. Association between interleukin 37 (rs3811047) polymorphism and multiple autoimmune diseases in a Chinese population: A PRISMA-compliant meta-analysis. Med (Baltim). 2018;97(15):e0386. doi:https://doi.org/10.1097/MD.0000000000010386.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection and analysis were performed by Basant Mahmoud, Adel Abdel-Moneim, and Rania Mahmoud. The first draft of the manuscript was written by Basant Mahmoud, Adel Abdel-Moneim, and Gamal Allam, and all authors commented on the previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Written consent was obtained from each participant before participating in the experiment, and the study was approved by the institutional ethics committee (Ref: NNI/FS/16/4).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdel-Moneim, A., Mahmoud, R., Allam, G. et al. Relationship between Cytokines and Metabolic Syndrome Components: Role of Pancreatic-Derived Factor, Interleukin-37, and Tumor Necrosis Factor-α in Metabolic Syndrome Patients. Ind J Clin Biochem 39, 37–46 (2024). https://doi.org/10.1007/s12291-022-01079-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-022-01079-z