Abstract
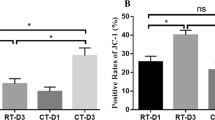
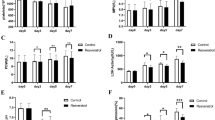
Platelets undergo remarkable morphological changes during storage. Platelets change into different sizes and densities and differ in their biochemistry and functions. However, the correlation between structural heterogeneity and platelet autophagy is largely unknown. The aim of this study was to investigate the autophagy process in vitro, such as routine storage of platelets, and explore the role of reactive oxygen species (ROS) involved in the regulation of platelet autophagy. The ROS and autophagy levels of platelet concentrates from apheresis platelets were evaluated through flow cytometry. The expression levels of autophagy-associated proteins (LC3I, LC3II, Beclin1, Parkin, and PINK1) were measured via Western blot. All biomarkers were dynamically monitored for seven days. Moreover, the morphological characteristics of platelet morphology during storage were analyzed through transmission electron microscopy (TEM). Flow cytometry showed that the levels of total cell ROS and mitochondria ROS increased in the stored platelets. Together with the increase in mitochondrial ROS, the autophagy signal LC3 in the platelets was strongly amplified. The number of swollen platelets (large platelets) considerably increased, and that of autophagy signal LC3 was remarkably higher than that of the normal platelets. Western blot revealed that the expression levels of Beclin1 and LC3 II/LC3 I ratio were enhanced, whereas those of Parkin and PINK1 almost did not change during the seven days of storage. The existence of autophagosomes or autophagolysosomes in the platelets at the middle stage of platelet storage was observed via TEM. Our data demonstrated that the subpopulation of large (swollen) platelets exhibited different autophagy patterns. Furthermore, increased platelet autophagy was associated with mitochondrial ROS. These preliminary results suggest that swelling platelets have a higher autophagy pattern than normal platelets during storage.







Similar content being viewed by others
References
Jurk K, Kehre BE (2005) Platelets: physiology and biochemistry. Semin Thromb Hemost 31(4):381–392
Andrews RK, Berndt MC (2004) Platelet physiology and thrombosis. Thromb Res 114(5–6):447–453
Devine DV, Serrano K (2010) The platelet storage lesion. Clin Lab Med 30(2):475–487
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26(7):1749–1760
Ghasemzadeh M, Hosseini E (2017) Platelet granule release is associated with reactive oxygen species generation during platelet storage: a direct link between platelet pro-inflammatory and oxidation states. Thromb Res 156:101–104
Vucic M, Stanojkovic Z, Antic A, Vucic J, Pavlovic V (2018) Evaluation of platelet activation in leukocyte-depleted platelet concentrates during storage. Bosn J Basic Med Sci 18(1):29–34
Feng W, Chang C, Luo D, Su H, Yu S, Hua W, Chen Z, Hu H, Liu W (2014) Dissection of autophagy in human platelets. Autophagy 10(4):642–651
Ohto H, Nollet KE (2011) Overview on platelet preservation: better controls over storage lesion. Transfus Apher Sci 44(3):321–325
Ouseph MM, Huang Y, Banerjee M, Joshi S, MacDonald L, Zhong Y, Liu H, Li X, Xiang B, Zhang G, Komatsu M, Yue Z, Li Z, Storrie B, Whiteheart SW, Wang QJ (2015) Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis. Blood 126(10):1224–1233
Cao Y, Cai J, Zhang S, Yuan N, Li X, Fang Y, Song L, Shang M, Liu S, Zhao W, Hu S, Wang J (2015) Loss of autophagy leads to failure in megakaryopoiesis, megakaryocyte differentiation, and thrombopoiesis in mice. Exp Hematol 43:488–494
Lee SH, Du J, Stitham J, Atteya G, Lee S, Xiang Y, Wang D, Jin Y, Leslie K, Spollett G, Srivastava A, Mannam P, Ostriker A, Martin KA, Tang WH, Hwa J (2016) Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol Med 8(7):779–795
Tanida I, Ueno T, Kominami E (2008) LC3 and autophagy. Methods Mol Biol 445:77–88
Tran S, Fairlie WD, Lee EF (2021) BECLIN1: protein structure, function and regulation. Cells 10(6):1522
Chen X, Wang Q, Li S, Li XJ, Yang W (2022) Mitochondrial-dependent and independent functions of PINK1. Front Cell Dev Biol 10:954536
Mangalpally KK, Siqueiros-Garcia A, Vaduganathan M, Dong JF, Kleiman NS, Guthikonda S (2010) Platelet activation patterns in platelet size sub-populations: differential responses to aspirin in vitro. J Thromb Thrombolysis 30(3):251–262
Eng KE, Panas MD, Karlsson Hedestam GB, McInerney GM (2010) A novel quantitative flow cytometry-based assay for autophagy. Autophagy 6(5):634–641
Neumüller J, Meisslitzer-Ruppitsch C, Ellinger A, Pavelkaa M, Jungbauerb C, Renzb R, Leitnerc G, Wagner T (2013) Monitoring of platelet activation in platelet concentrates using transmission electron microscopy. Transfus Med Hemother 40(2):101–107
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441(2):523–540
Luo XL, Jiang JY, Huang Z, Chen LX (2019) Autophagic regulation of platelet biology. J Cell Physiol 234(9):14483–14488
Al Amir Dache Z, Otandault A, Tanos R, Pastor B, Meddeb R, Sanchez C, Arena G, Lasorsa L, Bennett A, Grange T, El Messaoudi S, Mazard T, Prevostel C, Thierry AR (2020) Blood contains circulating cell-free respiratory competent mitochondria. FASEB J 34(3):3616–3630
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, Zhang Y, Han T, Ren J, Cao F, Chen Y (2017) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res 63(4):e12438
Zhang W, Ren H, Xu C, Zhu C, Wu H, Liu D, Wang J, Liu L, Li W, Ma Q, Du L, Zheng M, Zhang C, Liu J, Chen Q (2016) Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife 5:e21407
Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ (2010) Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465(7300):942–946
Portal-Núñez S, Esbrit P, Alcaraz MJ, Largo R (2016) Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem Pharmacol 108:1–10
Tang H, Gao M, Fu Y, Gui R, Ma X (2020) The effect of autophagic activity on the function of apheresis platelets and on the efficacy of clinical platelet transfusion. Transfus Med Hemother 47(4):302–313
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H (2014) p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 344(2):174–179
Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh-Armstrong N (2014) Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A 111(26):9633–9638
Torralba D, Baixauli F, Sánchez-Madrid F (2016) Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front Cell Dev Biol 4:107
McWilliams TG, Barini E, Pohjolan-Pirhonen R, Brooks SP, Singh F, Burel S, Balk K, Kumar A, Montava-Garriga L, Prescott AR, Hassoun SM, Mouton-Liger F, Ball G, Hills R, Knebel A, Ulusoy A, Di Monte DA, Tamjar J, Antico O, Fears K, Smith L, Brambilla R, Palin E, Valori M, Eerola-Rautio J, Tienari P, Corti O, Dunnett SB, Ganley IG, Suomalainen A, Muqit MMK (2018) Phosphorylation of Parkin at serine 65 is essential for its activation in vivo. Open Biol 8(11):180108
Okatsu K, Oka T, Iguchi M, Imamura K, Kosako H, Tani N, Kimura M, Go E, Koyano F, Funayama M, Shiba-Fukushima K, Sato S, Shimizu H, Fukunaga Y, Taniguchi H, Komatsu M, Hattori N, Mihara K, Tanaka K, Matsuda N (2012) PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nat Commun. 3:1016
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535(7613):551–555
Jiao H, Jiang D, Hu X, Du W, Ji L, Yang Y, Li X, Sho T, Wang X, Li Y, Wu YT, Wei YH, Hu X, Yu L (2021) Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell 184(11):2896–2910
Marcoux G, Duchez AC, Rousseau M, Lévesque T, Boudreau LH, Thibault L, Boilard E (2017) Microparticle and mitochondrial release during extended storage of different types of platelet concentrates. Platelets 28(3):272–280
Lesyk G, Jurasz P (2019) Advances in platelet subpopulation research. Front Cardiovasc Med 6:138
Ghasemzadeh M, Hosseini E, Roudsari O, Zadkhak P (2018) Intraplatelet reactive oxygen species (ROS) correlate with the shedding of adhesive receptors, microvesiculation and platelet adhesion to collagen during storage: does endogenous ROS generation downregulate platelet adhesive function? Thromb Res 163:153–161
Hosseini E, Hojjati S, Afzalniaye Gashti S, Ghasemzadeh M (2020) Collagen-dependent platelet dysfunction and its relevance to either mitochondrial ROS or cytosolic superoxide generation: a question about the quality and functional competence of long-stored platelets. Thromb J 18(1):18
Tyagi T, Jain K, Gu S, Qiu M, Gu V, Melchinger H, Rinder H, Martin K, Gardiner E, Lee A, Tang W, Hwa J (2022) A guide to molecular and functional investigations of platelets to bridge basic and clinical sciences. Nat Cardiovasc Res 1:223–237
Vučetić D, Ilić V, Vojvodić D, Subota V, Todorović M, Balint B (2018) Flow cytometry analysis of platelet populations: usefulness for monitoring the storage lesion in pooled buffy-coat platelet concentrates. Blood Transfus 16(1):83–92
Funding
We thank Yan Wang and Huihui Feng for contribution to serve the blood donors and PCs collection. This work was supported by grants from Natural Science Foundation of Ningbo (No.2019A610273 and No.2019A610268); Ningbo Public welfare science and technology project (No.2019C50083); Natural Science Foundation of Zhejiang Province (No.LY20H290006); Open Foundation of key laboratory of blood safety research of zhejiang province (No.2018KF010).
Author information
Authors and Affiliations
Contributions
GD and QL conceived and designed the experiments; LY, SY and YH performed the experiments and evaluated the results; LY and QL wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, L., Yu, S., He, Y. et al. High Autophagy Patterns in Swelling Platelets During Apheresis Platelet Storage. Indian J Hematol Blood Transfus 39, 670–678 (2023). https://doi.org/10.1007/s12288-023-01638-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-023-01638-1