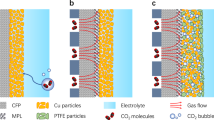
Abstract
The electrocatalytic reduction of CO2 presents a promising strategy in addressing environmental and energy crisis. Significant progress has been achieved via CO2 gas diffusion electrolysis, to react at high selectivity and high rate. However, the gas diffusion layer (GDL) of the gas diffusion electrode (GDE) still suffers from low tolerance and limited active sites. Here, the hydrophobic 1-octadecanethiol molecular was functionalized over the Cu catalyst layer of the GDE, which simultaneously stabilizes the GDL and exposes abundant active solid-liquid-gas three-phase interfaces. The resultant GDE exhibits multi-carbon (C2+) product selectivity over faradaic efficiency (FE) of 70.0% in the range of 100 to 800 mA·cm−2, with the peak FEC2+ of 85.2% at 800 mA·cm−2. Notably, the strengthened GDE could continuously drive high-current electrolysis for more than 100 h without flooding. This work opens a new way to improve CO2 gas diffusion electrolysis via surface molecular engineering.

Similar content being viewed by others
References
Zheng, Y.; Vasileff, A.; Zhou, X. L.; Jiao, Y.; Jaroniec, M.; Qiao, S. Z. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 2019, 141, 7676–7659.
Fan, L.; Xia, C.; Yang, F. Q.; Wang, J.; Wang, H. T.; Lu, Y. Y. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 2020, 6, eaay3111.
Zhong, H. X.; Meng, F. L.; Zhang, Q.; Liu, K. H.; Zhang, X. B. Highly efficient and selective CO2 electro-reduction with atomic Fe-C-N hybrid coordination on porous carbon nematosphere. Nano Res. 2019, 12, 2318–2323.
De Luna, P.; Quintero-Bermudez, R.; Dinh, C. T.; Ross, M. B.; Bushuyev, O. S.; Todorović, P.; Regier, T.; Kelley, S. O.; Yang, P. D.; Sargent, E. H. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110.
Cai, Z.; Zhang, Y. S.; Zhao, Y. X.; Wu, Y. S.; Xu, W. W; Wen, X. M.; Zhong, Y.; Zhang, Y.; Liu, W.; Wang, H. L. et al. Selectivity regulation of CO2 electroreduction through contact interface engineering on superwetting Cu nanoarray electrodes. Nano Res. 2019, 12, 345–349.
Lee, S. Y.; Jung, H.; Kim, N. K.; Oh, H. S.; Min, B. K.; Hwang, Y. J. Mixed copper states in anodized Cu electrocatalyst for stable and selective ethylene production from CO2 reduction. J. Am. Chem. Soc. 2018, 140, 8681–8689.
Lum, Y.; Ager, J. W. Stability of residual oxides in oxide-derived copper catalysts for electrochemical CO2 reduction investigated with 18O labeling. Angew. Chem., Int. Ed. 2018, 130, 560–563.
Tang, C.; Shi, J. J.; Bai, X. W.; Hu, A. Q.; Xuan, N. N.; Yue, Y. W.; Ye, T.; Liu, B.; Li, P. X.; Zhuang, P. Y. et al. CO2 reduction on copper’s twin boundary. ACS Catal. 2020, 10, 2026–2032.
Yang, Y.; Luo, M. C.; Zhang, W. Y.; Sun, Y. J.; Chen, X.; Guo, S. J. Metal surface and interface energy electrocatalysis: Fundamentals, performance engineering, and opportunities. Chem 2018, 4, 2054–2083.
Wang, W.; Shang, L.; Chang, G. J.; Yan, C. Y.; Shi, R.; Zhao, Y. X.; Waterhouse, G. I. N.; Yang, D. J.; Zhang, T. R. Intrinsic carbon-defect-driven electrocatalytic reduction of carbon dioxide. Adv. Mater. 2019, 31, 1808276.
Higgins, D.; Hahn, C.; Xiang, C. X.; Jaramillo, T. F.; Weber, A. Z. Gas-diffusion electrodes for carbon dioxide reduction: A new paradigm. ACS Energy Lett. 2019, 4, 317–324.
Wakerley, D.; Lamaison, S.; Ozanam, F.; Menguy, N.; Mercier, D.; Marcus, P.; Fontecave, M.; Mougel, V. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019, 18, 1222–1227.
Ren, W. H.; Zhao, C. Paths towards enhanced electrochemical CO2 reduction. Nat. Sci. Rev. 2020, 7, 7–9.
Weekes, D. M.; Salvatore, D. A.; Reyes, A.; Huang, A. X.; Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 2018, 51, 910–918.
Lu, X.; Zhu, C. Q.; Wu, Z. S.; Xuan, J.; Francisco, J. S.; Wang, H. L. In situ observation of the pH gradient near the gas diffusion electrode of CO2 reduction in alkaline electrolyte. J. Am. Chem. Soc. 2020, 142, 15438–15444.
Yang, K. L.; Kas, R.; Smith, W. A.; Burdyny, T. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2 reduction. ACS Energy Lett. 2021, 6, 33–40.
Forner-Cuenca, A.; Biesdorf, J.; Gubler, L.; Kristiansen, P. M.; Schmidt, T. J.; Boillat, P. Engineered water highways in fuel cells: Radiation grafting of gas diffusion layers. Adv. Mater. 2015, 27, 6317–6322.
Jeanty, P.; Scherer, C.; Magori, E.; Wiesner-Fleischer, K.; Hinrichsen, O.; Fleischer, M. Upscaling and continuous operation of electrochemical CO2 to CO conversion in aqueous solutions on silver gas diffusion electrodes. J. CO2 Util. 2018, 24, 454–462.
De Mot, B.; Hereijgers, J.; Duarte, M.; Breugelmans, T. Influence of flow and pressure distribution inside a gas diffusion electrode on the performance of a flow-by CO2 electrolyzer. Chem. Eng. J. 2019, 378, 122224.
Leonard, M. E.; Clarke, L. E.; Forner-Cuenca, A.; Brown, S. M.; Brushett, F. R. Investigating electrode flooding in a flowing electrolyte, gas-fed carbon dioxide electrolyzer. ChemSusChem 2020, 13, 400–411.
Shi, R.; Guo, J. H.; Zhang, X. R.; Waterhouse, G. I. N.; Han, Z. J.; Zhao, Y. X.; Shang, L.; Zhou, C.; Jiang, L.; Zhang, T. R. Efficient wettability-controlled electroreduction of CO2 to CO at Au/C interfaces. Nat. Commun. 2020, 11, 3028.
Dinh, C. T.; Burdyny, T.; Kibria, M. G.; Seifitokaldani, A.; Gabardo, C. M.; de Arquer, F. P. G.; Kiani, A.; Edwards, J. P.; De Luna, P.; Bushuyev, O. S. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360, 783–787.
Xing, Z.; Hu, L.; Ripatti, D. S.; Hu, X.; Feng, X. F. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 2021, 12, 136.
Wicks, J.; Jue, M. L.; Beck, V. A.; Oakdale, J. S.; Dudukovic, N. A.; Clemens, A. L.; Liang, S. W.; Ellis, M. E.; Lee, G.; Baker, S. E. et al. 3D-printable fluoropolymer gas diffusion layers for CO2 electroreduction. Adv. Mater. 2021, 33, 2003855.
Ma, W. C.; Xie, S. J.; Liu, T. T.; Fan, Q. Y.; Ye, J. Y.; Sun, F. F.; Jiang, Z.; Zhang, Q. H.; Cheng, J.; Wang, Y. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C-C coupling over fluorine-modified copper. Nat. Catal. 2020, 3, 478–487.
Yang, W. J.; Li, T. Q.; Zhou, H. H.; Huang, Z.; Fu, C. P.; Chen, L.; Li, M. B.; Kuang, Y. F. Electrochemical and anti-corrosion properties of octadecanethiol and benzotriazole binary self-assembled monolayers on copper. Electrochim. Acta 2016, 220, 245–251.
Zhao, H. Y.; Jin, J.; Tian, W. J.; Li, R.; Yu, Z.; Song, W.; Cong, Q.; Zhao, B.; Ozaki, Y. Three-dimensional superhydrophobic surface-enhanced Raman spectroscopy substrate for sensitive detection of pollutants in real environments. J. Mater. Chem. A 2015, 3, 4330–4337.
Ying, T. An advanced anti-tarnish process for silver coins and silverware—monomolecular octadecanethiol protective film. Tribol. Trans. 2021, 64, 341–349.
Tetzner, K.; Schroder, K. A.; Bock, K. Photonic curing of sol-gel derived HfO2 dielectrics for organic field-effect transistors. Ceram. Int. 2014, 40, 15753–15761.
Liu, J. H.; Yang, L. M.; Ganz, E. Electrochemical reduction of CO2 by single atom catalyst TM-TCNQ monolayers. J. Mater. Chem. A 2019, 7, 3805–3814.
Shinagawa, T.; Larrazábal, G. O.; Martín, A. J.; Krumeich, F.; Pérez-Ramírez, J. Sulfur-modified copper catalysts for the electrochemical reduction of carbon dioxide to formate. ACS Catal. 2018, 8, 837–844.
Ahn, S.; Klyukin, K.; Wakeham, R. J.; Rudd, J. A.; Lewis, A. R.; Alexander, S.; Carla, F.; Alexandrov, V.; Andreoli, E. Poly-amide modified copper foam electrodes for enhanced electrochemical reduction of carbon dioxide. ACS Catal. 2018, 8, 4132–4142.
Wei, X.; Yin, Z. L.; Lyu, K. J.; Li, Z.; Gong, J.; Wang, G. W.; Xiao, L.; Lu, J. T.; Zhuang, L. Highly selective reduction of CO2 to C2+ hydrocarbons at copper/polyaniline interfaces. ACS Catal. 2020, 10, 4103–4111.
Zhu, M. H.; Chen, J. C.; Huang, L. B.; Ye, R. Q.; Xu, J.; Han, Y. F. Covalently grafting cobalt porphyrin onto carbon nanotubes for efficient CO2 electroreduction. Angew. Chem., Int. Ed. 2019, 131, 6667–66671.
Peykov, V.; Quinn, A.; Ralston, J. Electrowetting: A model for contact-angle saturation. Colloid Polym. Sci. 2000, 278, 789–793.
Löwe, A.; Rieg, C.; Hierlemann, T.; Salas, N.; Kopljar, D.; Wagner, N.; Klemm, E. Influence of temperature on the performance of gas diffusion electrodes in the CO2 reduction reaction. ChemElectroChem 2019, 6, 4497–4506.
Zhong, M.; Tran, K.; Min, Y. M.; Wang, C. H.; Wang, Z. Y.; Dinh, C. T.; De Luna, P.; Yu, Z. Q.; Rasouli, A. S.; Brodersen, P. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 2020, 581, 178–183.
Acknowledgements
This work was financially supported by the International (Regional) Cooperation and Exchange Projects of the National Natural Science Foundation of China (No. 51920105003), the National Natural Science Funds for Distinguished Young Scholars (No. 51725201), the Innovation Program of Shanghai Municipal Education Commission (No. E00014), the National Natural Science Foundation of China (Nos. 51902105 and 22072045), the Shanghai Engineering Research Center of Hierarchical Nanomaterials (No. 18DZ2252400) and the Shanghai Sailing Program (No. 19YF1411600). The authors acknowledge the support by Shanghai Rising-star and Shuguang Programs (Nos. 20QA1402400 and 17SG30), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning. Additional support was provided by the Feringa Nobel Prize Scientist Joint Research Center. The authors thank the Frontiers Science Center for Materiobiology and Dynamic Chemistry. The authors also thank the crew of the 1W1B beamline of Beijing Synchrotron Radiation Facility (BSRF) for their constructive assistance with the XAFS measurements and data analyses.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2021_3675_MOESM1_ESM.pdf
Hydrophobic 1-octadecanethiol functionalized copper catalyst promotes robust high-current CO2 gas-diffusion electrolysis
Rights and permissions
About this article
Cite this article
Xue, L., Wu, X., Liu, Y. et al. Hydrophobic 1-octadecanethiol functionalized copper catalyst promotes robust high-current CO2 gas-diffusion electrolysis. Nano Res. 15, 1393–1398 (2022). https://doi.org/10.1007/s12274-021-3675-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3675-6