Abstract
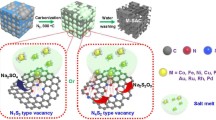
The development of low-cost and high-active cocatalysts is one of the most significant links for photocatalytic water splitting. Herein, a novel strategy of electron delocalization modulation for transition metal sulfides has been developed by anion hybridization. P-modified CoS2 (CoS2∣P) nanocrystals were firstly fabricated via a gas-solid reaction and coupled with CdS nanorods to construct a composite catalyst for solar H2 evolution reaction (HER). The CdS/CoS2∣P catalyst shows an HER rate of 57.8 µmol·h−1, which is 18 times that of the bare CdS, 8 times that of the CdS/CoS2, and twice that of Pt/CdS. The reduced energy barrier and suppressed reverse reaction for HER on the catalyst have been predicted and explained by density functional theory (DFT) calculation. The underlying design strategy of novel cocatalysts by electron delocalization modulation may shed light on the rational development of other advanced catalysts for energy conversion.

Similar content being viewed by others
References
Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2020, 120, 919–985.
Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399.
Meng, A. Y.; Zhang, L. Y.; Cheng, B.; Yu, J. G. Dual cocatalysts in TiO2 photocatalysis. Adv. Mater. 2019, 31, 1807660.
Sun, S. C.; Zhang, X. Y.; Liu, X. L.; Pan, L.; Zhang, X. W.; Zou, J. J. Design and construction of cocatalysts for photocatalytic water splitting. Acta Phys. Chim. Sin. 2020, 36, 1905007.
Ran, J. J.; Zhang, J.; Yu, J. G.; Jaroniec, M.; Qiao, S. Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812.
Zhang, H. B.; Zhang, P.; Qiu, M.; Dong, J. C.; Zhang, Y. F.; Lou, X. W. Ultrasmall MoOx clusters as a novel cocatalyst for photocatalytic hydrogen evolution. Adv. Mater. 2019, 31, 1804883.
Li, L. H.; Deng, Z. X.; Yu, L. L.; Lin, Z. Y.; Wang, W. L.; Yang, G. W. Amorphous transitional metal borides as substitutes for Pt cocatalysts for photocatalytic water splitting. Nano Energy 2016, 27, 103–113.
Sun, Z. J.; Zheng, H. F.; Li, J. S.; Du, P. W. Extraordinarily efficient photocatalytic hydrogen evolution in water using semiconductor nanorods integrated with crystalline Ni2P cocatalysts. Energy Environ. Sci. 2015, 8, 2668–2676.
Zhang, J. Y.; Liu, Y. C.; Sun, C. Q.; Xi, P. X.; Peng, S. L.; Gao, D. Q.; Xue, D. S. Accelerated hydrogen evolution reaction in CoS2 by transition-metal doping. ACS Energy Lett. 2018, 3, 779–786.
Yin, J.; Jin, J.; Zhang, H.; Lu, M.; Peng, Y.; Huang, B. L.; Xi, P. X.; Yan, C. H. Atomic arrangement in metal-doped NiS2 boosts the hydrogen evolution reaction in alkaline media. Angew. Chem., Int. Ed. 2019, 58, 18676–18682.
Chang, K.; Hai, X.; Ye, J. H. Transition metal disulfides as noble-metal-alternative co-catalysts for solar hydrogen production. Adv. Energy Mater. 2016, 6, 1502555.
Chang, K.; Pang, H.; Hai, X.; Zhao, G. X.; Zhang, H. B.; Shi, L.; Ichihara, F.; Ye, J. H. Ultra-small freestanding amorphous molybdenum sulfide colloidal nanodots for highly efficient photocatalytic hydrogen evolution reaction. Appl. Catal. B Environ. 2018, 232, 446–453.
Zong, X.; Yan, H. J.; Wu, G. P.; Ma, G. J.; Wen, F. Y.; Wang, L.; Li, C. Enhancement of photocatalytic H2 evolution on CdS by loading MoS2 as cocatalyst under visible light irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177.
Shi, Y. M.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541.
Carenco, S.; Portehault, D.; Boissière, C.; Mézailles, N.; Sanchez, C. Nanoscaled metal borides and phosphides: Recent developments and perspectives. Chem. Rev. 2013, 113, 7981–8065.
Liu, P.; Rodriguez, J. A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P (001) surface: The importance of ensemble effect. J. Am. Chem. Soc. 2005, 127, 14871–14878.
Xiao, P.; Sk, M. A.; Thia, L.; Ge, X. M.; Lim, R. J.; Wang, J. Y.; Lim, K. H.; Wang, X. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 2624–2629.
Cabán-Acevedo, M.; Stone, M. L.; Schmidt, J. R.; Thomas, J. G.; Ding, Q.; Chang, H. C.; Tsai, M. L.; He, J. H.; Jin, S. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 2015, 14, 1245–1251.
Zhang, X.; Luo, Z. M.; Yu, P.; Cai, Y. Q.; Du, Y. H.; Wu, D. X.; Gao, S.; Tan, C. L.; Li, Z.; Ren, M. Q. et al. Lithiation-induced amorphization of Pd3P2S8 for highly efficient hydrogen evolution. Nat. Catal. 2018, 1, 460–468.
Liu, W.; Hu, E. Y.; Jiang, H.; Xiang, Y. J.; Weng, Z.; Li, M.; Fan, Q.; Yu, X. Q.; Altman, E. I.; Wang, H. L. A highly active and stable hydrogen evolution catalyst based on pyrite-structured cobalt phosphosulfide. Nat. Commun. 2016, 7, 10771.
Zheng, J. M.; Tian, J.; Wu, D. X.; Gu, M.; Xu, W.; Wang, C. M.; Gao, F.; Engelhard, M. H.; Zhang, J. G.; Liu, J. et al. Lewis acid-base interactions between polysulfides and metal organic framework in lithium sulfur batteries. Nano Lett. 2014, 14, 2345–2352.
Zhang, S.; Huang, Z. Q.; Ma, Y. Y.; Gao, W.; Li, J.; Cao, F. X.; Li, L.; Chang, C. R.; Qu, Y. Solid frustrated-lewis-pair catalysts constructed by regulations on surface defects of porous nanorods of CeO2. Nat. Commun. 2017, 8, 15266.
Huang, Z. Q.; Liu, L. P.; Qi, S. T.; Zhang, S.; Qu, Y. Q.; Chang, C. R. Understanding All-solid frustrated-Lewis-pair sites on CeO2 from theoretical perspectives. ACS Catal. 2018, 8, 546–554.
Liu, J. C.; Ma, X. L.; Li, Y.; Wang, Y. G.; Xiao, H.; Li, J. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 2018, 9, 1610.
Wang, Y.; Zhuo, H. Y.; Zhang, X.; Dai, X. P.; Yu, K. M.; Luan, C. L.; Yu, L.; Xiao, Y.; Li, J.; Wang, M. L. et al. Synergistic effect between undercoordinated platinum atoms and defective nickel hydroxide on enhanced hydrogen evolution reaction in alkaline solution. Nano Energy 2018, 48, 590–599.
Zou, X. X.; Wu, Y. Y.; Liu, Y. P.; Liu, D. P.; Li, W.; Gu, L.; Liu, H.; Wang, P. W.; Sun, L.; Zhang, Y. In situ generation of bifunctional, efficient Fe-based catalysts from mackinawite iron sulfide for water splitting. Chem 2018, 4, 1139–1152.
Yan, H. J.; Xie, Y.; Wu, A. P.; Cai, Z. C.; Wang, L.; Tian, C. G.; Zhang, X. M.; Fu, H. G. Anion-modulated HER and OER activities of 3D Ni-V-based interstitial compound heterojunctions for high-efficiency and stable overall water splitting. Adv. Mater. 2019, 31, 1901174.
Cheng, T.; Wang, L.; Merinov, B. V.; Goddard III, W. A. Explanation of dramatic pH-dependence of hydrogen binding on noble metal electrode: Greatly weakened water adsorption at high pH. J. Am. Chem. Soc. 2018, 140, 7787–7790.
Kim, Y. D.; Chang, Y. C.; Klein, M. V. Effect of d electrons in transition-metal ions on band-gap energies of diluted magnetic semiconductors. Phys. Rev. B 1993, 48, 17770–17775.
Savin, A.; Nesper, R.; Wengert, S.; Fässler, T. F. ELF: The electron localization function. Angew. Chem., Int. Ed. 1997, 36, 1808–1832.
Huang, H. M.; Dai, B. Y.; Wang, W.; Lu, C. H.; Kou, J. H.; Ni, Y. R.; Wang, L. Z.; Xu, Z. Z. Oriented built-in electric field introduced by surface gradient diffusion doping for enhanced photocatalytic H2 evolution in CdS nanorods. Nano Lett. 2017, 17, 3803–3808.
Cordero, B.; Gómez, V.; Platero-Prats, A. E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 2832–2838.
Yan, Y.; Liu, C. Y.; Jian, H. W.; Cheng, X.; Hu, T.; Wang, D.; Shang, L.; Chen, G.; Schaaf, P.; Wang, X. Y. et al. Substitutionally dispersed high-oxidation CoOx clusters in the lattice of rutile TiO2 triggering efficient Co-Ti cooperative catalytic centers for oxygen evolution reactions. Adv. Funct. Mater. 2020, 31, 2009610.
Regulacio, M. D.; Ye, C.; Lim, S. H.; Bosman, M.; Polavarapu, L.; Koh, W. L.; Zhang, J.; Xu, Q. H.; Han, M. Y. One-pot synthesis of Cu1.94S-CdS and Cu1.94S−ZnxCd1−xS nanodisk heterostructures. J. Am. Chem. Soc. 2011, 133, 2052–2055.
Peng, S. J.; Li, L. L.; Han, X. P.; Sun, W. P.; Srinivasan, M.; Mhaisalkar, S. G.; Cheng, F. Y.; Yan, Q. Y.; Chen, J.; Ramakrishna, S. Cobalt sulfide nanosheet/graphene/carbon nanotube nanocomposites as flexible electrodes for hydrogen evolution. Angew. Chem., Int. Ed. 2014, 53, 12594–12599.
Shang, L.; Tong, B.; Yu, H. J.; Waterhouse, G. I. N.; Zhou, C.; Zhao, Y. F.; Tahir, M.; Wu, L. Z.; Tung, C. H.; Zhang, T. R. CdS nanoparticle-decorated Cd nanosheets for efficient visible light-driven photocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1501241.
Yuan, J. L.; Wen, J. Q.; Zhong, Y. M.; Li, X.; Fang, Y. P.; Zhang, S. S.; Liu, W. Enhanced photocatalytic H2 evolution over noble-metal-free NiS cocatalyst modified CdS nanorods/g-C3N4 heterojunctions. J. Mater. Chem. A 2015, 3, 18244–18255.
Liu, J. C.; Wang, Y. G.; Li, J. Toward rational design of oxide-supported single-atom catalysts: Atomic dispersion of gold on ceria. J. Am. Chem. Soc. 2017, 139, 6190–6199.
Zhao, Z. Y. Single water molecule adsorption and decomposition on the low-index stoichiometric rutile TiO2 surfaces. J. Phys. Chem. C 2014, 118, 4287–4295.
Camellone, M. F.; Fabris, S. Reaction mechanisms for the CO oxidation on Au/CeO2 catalysts: Activity of substitutional Au3+/Au+ cations and deactivation of supported Au+ adatoms. J. Am. Chem. Soc. 2009, 131, 10473–10483.
Wang, X. S.; Zheng, Y.; Sheng, W. C.; Xu, Z. J.; Jaroniec, M.; Qiao, S. Z. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125–138.
Zhao, L.; Zhang, Y.; Zhao, Z. L.; Zhang, Q. H.; Huang, L. B.; Gu, L.; Lu, G.; Hu, J. S.; Wan, L. J. Steering elementary steps towards efficient alkaline hydrogen evolution via size-dependent Ni/NiO nanoscale heterosurfaces. Natl. Sci. Rev. 2020, 7, 27–36.
Sun, K.; Zhao, L.; Zeng, L.; Liu, S.; Zhu, H.; Li, Y.; Chen, Z.; Zhuang, Z.; Li, Z.; Liu, Z. et al. Reaction environment self-modification on low-coordination Ni2+ octahedra atomic interface for superior electrocatalytic overall water splitting. Nano Res. 2020, 13, 3068–3074.
Li, F.; Han, G. F.; Noh, H. J.; Lu, Y. L.; Xu, J.; Bu, Y. F.; Fu, Z. P.; Baek, J. B. Construction of porous Mo3P/Mo nanobelts as catalysts for efficient water splitting. Angew. Chem., Int. Ed. 2018, 57, 14139–14143.
Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K. C.; Uchimura, M.; Paulikas, A. P.; Stamenkovic, V.; Markovic, N. M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+−Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260.
Zhou, K. L.; Wang, C. H.; Wang, Z. L.; Han, C. B.; Zhang, Q. Q.; Ke, X. X.; Liu, J. B.; Wang, H. Seamlessly conductive Co(OH)2 tailored atomically dispersed Pt electrocatalyst with a hierarchical nanostructure for an efficient hydrogen evolution reaction. Energy Environ. Sci. 2020, 13, 3082–3092.
Guo, Q.; Xu, C. B.; Ren, Z. F.; Yang, W. S.; Ma, Z. B.; Dai, D. X.; Fan, H. J.; Minton, T. K.; Yang, X. M. Stepwise photocatalytic dissociation of methanol and water on TiO2 (110). J. Am. Chem. Soc. 2012, 134, 13366–13373.
Luo, Z. Y.; Zhang, H.; Yang, Y. Q.; Wang, X.; Li, Y.; Jin, Z.; Jiang, Z.; Liu, C. P.; Xing, W.; Ge, J. J. Reactant friendly hydrogen evolution interface based on di-anionic MoS2 surface. Nat. Commun. 2020, 11, 1116.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51872138 and 22002060), Natural Science Foundation of Jiangsu Province (No. BK20181380), Qing Lan Project, Six Talent Peaks Project in Jiangsu Province (No. XCL-029) and Priority Academic Program Development of the Jiangsu Higher Education Institutions (PAPD). Dr. Hengming Huang gratefully acknowledges the support provided by China Scholarships Council (CSC No. 202008320109) and China Postdoctoral Science Foundation (No. 2020M681564). The authors are grateful to the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis (CMM), The University of Queensland, and the Queensland node of the Australian National Fabrication Facility. The computational resources generously provided by the High-Performance Computing Center of Nanjing Tech University are greatly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Huang, H., Xue, C., Fang, Z. et al. Bridging localized electron states of pyrite-type CoS2 cocatalyst for activated solar H2 evolution. Nano Res. 15, 202–208 (2022). https://doi.org/10.1007/s12274-021-3457-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3457-1