Abstract
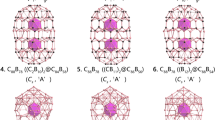
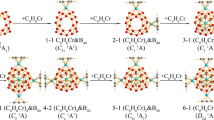
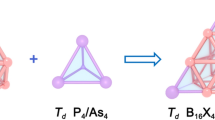
Boron allotropes are known to be predominately constructed by icosahedral B12 cages, while icosahedral-B12 stuffing proves to effectively improve the stability of fullerene-like boron nanoclusters in the size range between B98–B102. However, the thermodynamically most stable core-shell borospherenes with a B12 icosahedron at the center still remains unknown. Based on the structural motif of D5h C70 and extensive first-principles theory calculations, we predict herein the high-symmetry C5v B111+ (3) which satisfies the Wade’s n+1 and n+2 skeletal electron counting rules exactly and the approximately electron sufficient Cs B111 (4), Cs B112 (5), Cs B113 (6), and Cs B114 (7) which are the most stable neutral core-shell borospherenes with a B12 icosahedron at the center reported to date in the size range between B68–B130, with Cs B112 (5) being the thermodynamically most favorite species in the series. Detailed orbital and bonding analyses indicate that these spherically aromatic species all contain a negatively charged icosahedral B122− core at the center which exhibits typical superatomic behaviors in the electronic configuration of 1S21P61D101F8, with its dangling valences saturated by twelve radial B-B 2c-2e σ bonds between the B12 inner core and the B70 outer shell. The infrared (IR) and Raman spectra of the concerned species are computationally simulated to facilitate their future characterizations.

Similar content being viewed by others
References
Oganov, A. R.; Chen, J. H.; Gatti, C.; Ma, Y. Z.; Glass, C. W.; Liu, Z. X.; Yu, T.; Kurakevych, O. O.; Solozhenko, V. L. Ionic high-pressure form of elemental boron. Nature 2009, 457, 863–867.
Albert, B.; Hillebrecht, H. Boron: Elementary challenge for experimenters and theoreticians. Angew. Chem., Int. Ed. 2009, 48, 8640–8668.
Cotton, A. F.; Murillo, C. A.; Bochmann, M; Wilkinson, G. Advanced Inorganic Chemistry; 6th ed. Wiley: New York, 1999; pp 1355.
Wang, L. S. Photoelectron spectroscopy of size-selected boron clusters: From planar structures to borophenes and borospherenes. Int. Rev. Phys. Chem. 2016, 35, 69–142.
Jian, T.; Chen, X. N.; Li, S. D.; Boldyrev, A. I.; Li, J.; Wang, L. S. Probing the structures and bonding of size-selected boron and doped-boron clusters. Chem. Soc. Rev. 2019, 48, 3550–3591.
Chen, Q.; Li, W. L.; Zhao, Y. F.; Hu, H. S.; Bai, H.; Li, H. R.; Tian, W. J.; Lu, H. G; Zhai, H. J.; Li, S. D. et al. Experimental and theoretical evidence of an axially chiral borospherene. ACS Nano 2015, 9, 754–760.
Zhai, H. J.; Zhao, Y. F.; Li, W. L.; Chen, Q.; Bai, H.; Hu, H. S.; Piazza, Z. A.; Tian, W. J.; Lu, H. G.; Wu, Y. B. et al. Observation of an all-boron fullerene. Nat. Chem. 2014, 6, 727–731.
Bai, H.; Chen, T. T.; Chen, Q.; Zhao, X. Y.; Zhang, Y. Y.; Chen, W. J.; Li, W. L.; Cheung, L. F.; Bai, B.; Cavanagh, J. et al. Planar B41− and B42− clusters with double-hexagonal vacancies. Nanoscale 2019, 11, 23286–23295.
Chen, Q.; Zhang, S. Y.; Bai, H.; Tian, W. J.; Gao, T.; Li, H. R.; Miao, C. Q.; Mu, Y. W.; Lu, H. G.; Zhai, H. J. et al. Cage-like B41+ and B422+: New chiral members of the borospherene family. Angew. Chem., Int. Ed. 2015, 54, 8160–8164.
Chen, Q.; Li, H. R.; Miao, C. Q.; Wang, Y. J.; Lu, H. G.; Mu, Y. W.; Ren, G. M.; Zhai, H. J.; Li, S. D. Endohedral Ca@B38: Stabilization of a B382−borospherene dianion by metal encapsulation. Phys. Chem. Chem. Phys. 2016, 18, 11610–11615.
Tian, W. J.; Chen, Q.; Li, H. R.; Yan, M.; Mu, Y. W.; Lu, H. G.; Zhai, H. J.; Li, S. D. Saturn-like charge-transfer complexes Li4&B36, Li5&B36+, and Li6&B362+: Exohedral metalloborospherenes with a perfect cage-like B364− core. Phys. Chem. Chem. Phys. 2016, 18, 9922–9926.
Chen, Q.; Li, H. R.; Tian, W. J.; Lu, H. G.; Zhai, H. J.; Li, S. D. Endohedral charge-transfer complex Ga@B37−: Stabilization of a B373− borospherene trianion by metal-encapsulation. Phys. Chem. Chem. Phys. 2016, 18, 14186–14190.
Wang, Y. J.; Zhao, Y. F.; Li, W. L.; Jian, T.; Chen, Q.; You, X. R.; Ou, T.; Zhao, X. Y.; Zhai, H. J.; Li, S. D. et al. Observation and characterization of the smallest borospherene, B28− and B28. J. Chem. Phys. 2016, 144, 064307.
Li, H. R.; Jian, T.; Li, W. L.; Miao, C. Q.; Wang, Y. J.; Chen, Q.; Luo, X. M.; Wang, K.; Zhai, H. J.; Li, S. D. et al. Competition between quasi-planar and cage-like structures in the B29− cluster: Photoelectron spectroscopy and ab initio calculations. Phys. Chem. Chem. Phys. 2016, 18, 29147–29155.
Oger, E.; Crawford, N. R. M.; Kelting, R.; Weis, P.; Kappes, M. M.; Ahlrichs, R. Boron cluster cations: Transition from planar to cylindrical structures. Angew. Chem., Int. Ed. 2007, 46, 8503–8506.
Sai, L. W.; Wu, X.; Gao, N.; Zhao, J. J.; King, R. B. Boron clusters with 46, 48, and 50 atoms: Competition among the core-shell, bilayer and quasi-planar structures. Nanoscale 2017, 9, 13905–13909.
Pei, L.; Ma, Y. Y.; Yan, M.; Zhang, M.; Yuan, R. N.; Chen, Q.; Zan, W. Y.; Mu, Y. W.; Li, S. D. Bilayer B54, B60, and B62 clusters in a universal structural pattern. Eur. J. Inorg. Chem. 2020, 2020, 3296–3301.
Szwacki, N. G.; Sadrzadeh, A.; Yakobson, B. I. B80 fullerene: An ab initio prediction of geometry, stability, and electronic structure. Phys. Rev. Lett. 2007, 98, 166804.
De, S.; Willand, A.; Amsler, M.; Pochet, P.; Genovese, L.; Goedecker, S. Energy landscape of fullerene materials: A comparison of boron to boron nitride and carbon. Phys. Rev. Lett. 2011, 106, 225502.
Zhao, J. J.; Wang, L.; Li, F. Y.; Chen, Z. F. B80 and other medium-sized boron clusters: Core-shell structures, not hollow cages. J. Phys. Chem. A 2010, 114, 9969–9972.
Li, H.; Shao, N.; Shang, B.; Yuan, L. F.; Yang, J. L.; Zeng, X. C. Icosahedral B12-containing core-shell structures of B80. Chem. Commun. 2010, 46, 3878–3880.
Shang, B.; Yuan, L. F.; Zeng, X. C.; Yang, J. L. Ab initio prediction of amorphous B84. J. Phys. Chem. A 2010, 114, 2245–2249.
Prasad, D. L. V. K.; Jemmis, E. D. Stuffing improves the stability of fullerenelike boron clusters. Phys. Rev. Lett. 2008, 100, 165504.
Li, F. Y.; Jin, P.; Jiang, D. E.; Wang, L.; Zhang, S. B.; Zhao, J. J.; Chen, Z. F. B80 and B101−103 clusters: Remarkable stability of the core-shell structures established by validated density functionals. J. Chem. Phys. 2012, 136, 074302.
VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. QUICKSTEP: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128.
Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170.
Tao, J. M., Perdew, J. P.; Staroverov, V. N.; Scuseria, G. E. Climbing the density functional ladder: Nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 2003, 91, 146401.
Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627.
Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586.
Frisch, M. J. Gaussian 09, Revision D. 01, Gaussian Inc. Wallingford, CT, 2009.
Glendening, E. D.; Landis, C. R.; Weinhold, F. NBO 6.0: Natural bond orbital analysis program. J. Comput. Chem. 2013, 34, 1429–1437.
von Ragué Schleyer, P.; Maerker, C.; Dransfeld, A.; Jiao, H. J.; van Eikema Hommes, N. J. R. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318.
Chen, Z. F.; Wannere, C. S.; Corminboeuf, C.; Puchta, R.; von Ragué Schleyer, P. Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 2005, 105, 3842–3888.
Zubarev, D. Y.; Boldyrev, A. I. Developing paradigms of chemical bonding: Adaptive natural density partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207–5217.
Tkachenko, N. V.; Boldyrev, A. I. Chemical bonding analysis of excited states using the adaptive natural density partitioning method. Phys. Chem. Chem. Phys. 2019, 21, 9590–9596.
Zhang, B. L.; Wang, C. Z.; Ho, K. M.; Xu, C. H.; Chan, C. T. The geometry of small fullerene cages: C20 to C70. J. Chem. Phys. 1992, 97, 5007–5011.
Wade, K. The structural significance of the number of skeletal bonding electron-pairs in carboranes, the higher boranes and borane anions, and various transition-metal carbonyl cluster compounds. J. Chem. Soc. D. 1971, 15, 792–793.
Özdoğan, C.; Mukhopadhyay, S.; Hayami, W.; Güvenc, Z. B.; Pandey, R.; Boustani, I. The unusually stable B100 fullerene, structural transitions in boron nanostructures, and a comparative study of α- and γ-boron and sheets. J. Phys. Chem. C 2010, 114, 4362–4375.
Rahane, A. B.; Kumar V. B84: A quasi-planar boron cluster stabilized with hexagonal holes. Nanoscale 2015, 7, 4055–4062.
Ciuparu, D.; Klie, R. F.; Zhu, Y. M.; Pfefferle, L. Synthesis of pure boron single-wall nanotubes. J. Phys. Chem. B 2004, 108, 3967–3969.
Acknowledgements
The authors are grateful to professor B. I. Boldyrev and Dr. N. V. Tkachenko for their valuable help in AdNDP bonding analyses. This work was supported by the National Natural Science Foundation of China (Nos. 21720102006 and 21973057 to S.-D. Li and 21473106 to H.-G. Lu).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2021_3411_MOESM1_ESM.pdf
B111, B112, B113, and B114: The most stable core-shell borospherenes with an icosahedral B12 core at the center exhibiting superatomic behaviors
Rights and permissions
About this article
Cite this article
Zhang, M., Lu, HG. & Li, SD. B111, B112, B113, and B114: The most stable core-shell borospherenes with an icosahedral B12 core at the center exhibiting superatomic behaviors. Nano Res. 14, 4719–4724 (2021). https://doi.org/10.1007/s12274-021-3411-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3411-x