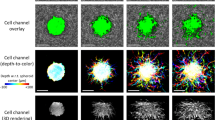
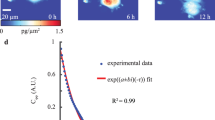
Abstract
The mechanical force between cells and the extracellular microenvironment is crucial to many physiological processes such as cancer metastasis and stem cell differentiation. Mitosis plays an essential role in all these processes and thus an in-depth understanding of forces during mitosis gains insight into disease diagnosis and disease treatment. Here, we develop a traction force microscope method based on monolayer fluorescent beads for measuring the weak traction force (tens of Pa) of mitotic cells in three dimensions. We quantify traction forces of human ovarian granulosa (KGN) cells exerted on the extracellular matrix throughout the entire cell cycle in three dimensions. Our measurements reveal how forces vary during the cell cycle, especially during cell division. Furthermore, we study the effect of paclitaxel (PTX) and nocodazole (NDZ) on mitotic KGN cells through the measurement of traction forces. Our results show that mitotic cells with high concentrations of PTX exert a larger force than those with high concentrations of NDZ, which proved to be caused by changes in the structure and number of microtubules. These findings reveal the key functions of microtubule in generating traction forces during cell mitosis and explain how dividing cells regulate themselves in response to anti-mitosis drugs. This work provides a powerful tool for investigating cell-matrix interactions during mitosis and may offer a potential way to new therapies for cancer.

Similar content being viewed by others
References
Preston-Martin, S.; Pike, M. C.; Ross, R. K.; Jones, P. A.; Henderson, B. E. Increased cell division as a cause of human cancer. Cancer Res. 1990, 50, 7415–7421.
Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801.
Papalazarou, V.; Zhang, T.; Paul, N. R.; Juin, A.; Cantini, M.; Maddocks, O. D. K.; Salmeron-Sanchez, M.; Machesky, L. M. The creatine-phosphagen system is mechanoresponsive in pancreatic adenocarcinoma and fuels invasion and metastasis. Nat. Metab. 2020, 2, 62–80.
Baker, B. M.; Chen, C. S. Deconstructing the third dimension — how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024.
Huang, S.; Ingber, D. E. Cell tension, matrix mechanics, and cancer development. Cancer Cell 2005, 8, 175–176.
Huebsch, N.; Arany, P. R.; Mao, A. S.; Shvartsman, D.; Ali, O. A.; Bencherif, S. A.; Rivera-Feliciano, J.; Mooney, D. J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526.
Munevar, S.; Wang, Y. L.; Dembo, M. Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys. J. 2001, 80, 1744–1757.
Jiang, G. Y.; Giannone, G.; Critchley, D. R.; Fukumoto, E.; Sheetz, M. P. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 2003, 424, 334–337.
Li, M.; Xi, N.; Wang, Y. C.; Liu, L. Q. Advances in atomic force microscopy for single-cell analysis. Nano Res. 2019, 12, 703–718.
Tan, J. L.; Tien, J.; Pirone, D. M.; Gray, D. S.; Bhadriraju, K.; Chen, C. S. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA 2003, 100, 1484–1489.
Harris, A. K.; Wild, P.; Stopak, D. Silicone rubber substrata: A new wrinkle in the study of cell locomotion. Science 1980, 208, 177–179.
Legant, W. R.; Choi, C. K.; Miller, J. S.; Shao, L.; Gao, L.; Betzig, E.; Chen, C. S. Multidimensional traction force microscopy reveals out-of-plane rotational moments about focal adhesions. Proc. Natl. Acad. Sci. USA 2013, 110, 881–886.
Style, R. W.; Boltyanskiy, R.; German, G. K.; Hyland, C.; MacMinn, C. W.; Mertz, A. F.; Wilen, L. A.; Xu, Y.; Dufresne, E. R. Traction force microscopy in physics and biology. Soft Matter 2014, 10, 4047–4055.
Dembo, M.; Wang, Y. L. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 1999, 76, 2307–2316.
Huang, J. Y.; Wang, L. L.; Xiong, C. Y.; Yuan, F. Elastic hydrogel as a sensor for detection of mechanical stress generated by single cells grown in three-dimensional environment. Biomaterials 2016, 98, 103–112.
Provenzano, P. P.; Keely, P. J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 2011, 124, 1195–1205.
Vianay, B.; Senger, F.; Alamos, S.; Anjur-Dietrich, M.; Bearce, E.; Cheeseman, B.; Lee, L.; Théry, M. Variation in traction forces during cell cycle progression. Biol. Cell 2018, 110, 91–96.
Panagiotakopoulou, M.; Lendenmann, T.; Pramotton, F. M.; Giampietro, C.; Stefopoulos, G.; Poulikakos, D.; Ferrari, A. Cell cycle-dependent force transmission in cancer cells. Mol. Biol. Cell 2018, 29, 2528–2539.
Nam, S.; Chaudhuri, O. Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments. Nat. Phys. 2018, 14, 621–628.
Jahan, G. S.; Yumura, S. Traction force and its regulation during cytokinesis in Dictyostelium cells. Eur. J. Cell Biol. 2017, 96, 515–528.
Tanimoto, H.; Sano, M. Dynamics of traction stress field during cell division. Phys. Rev. Lett. 2012, 109, 248110.
Lesman, A.; Notbohm, J.; Tirrell, D. A.; Ravichandran, G. Contractile forces regulate cell division in three-dimensional environments. J. Cell Biol. 2014, 205, 155–162.
Maskarinec, S. A.; Franck, C.; Tirrell, D. A.; Ravichandran, G. Quantifying cellular traction forces in three dimensions. Proc. Natl. Acad. Sci. USA 2009, 106, 22108–22113.
Hur, S. S.; Zhao, Y. H.; Li, Y. S.; Botvinick, E.; Chien, S. Live cells exert 3-dimensional traction forces on their substrata. Cell. Mol. Bioeng. 2009, 2, 425–436.
Pelham, R. J. Jr.; Wang, Y. L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665.
Marinković, A.; Mih, J. D.; Park, J. A.; Liu, F.; Tschumperlin, D. J. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L169–L180.
Denisin, A. K.; Pruitt, B. L. Tuning the range of polyacrylamide gel stiffness for Mechanobiology applications. ACS Appl. Mater. Interfaces 2016, 8, 21893–21902.
Gao, Y.; Cheng, T.; Su, Y.; Xu, X. H.; Zhang, Y.; Zhang, Q. C. High-efficiency and high-accuracy digital image correlation for three-dimensional measurement. Opt. Lasers Eng. 2015, 65, 73–80.
Su, Y.; Zhang, Q. C.; Xu, X. H.; Gao, Z. R. Quality assessment of speckle patterns for DIC by consideration of both systematic errors and random errors. Opt. Lasers Eng. 2016, 86, 132–142.
Xu, X. H.; Su, Y.; Cai, Y. L.; Cheng, T.; Zhang, Q. C. Effects of various shape functions and subset size in local deformation measurements using DIC. Exp. Mech. 2015, 55, 1575–1590.
Xu, X. Study on local deformation measurement accuracy and compensation methods in digital image correlation. Ph.D.Dissertation, University of Science and Technology of China, 2015.
Steinwachs, J.; Metzner, C.; Skodzek, K.; Lang, N.; Thievessen, I.; Mark, C.; Münster, S.; Aifantis, K. E.; Fabry, B. Three-dimensional force microscopy of cells in biopolymer networks. Nat. Methods 2016, 13, 171–176.
Landau, L. D.; Lifshitz, E. M. Theory of Elasticity; Pergamon: New York, 1986.
Lauffenburger, D. A.; Horwitz, A. F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369.
Ridley, A. J.; Schwartz, M. A.; Burridge, K.; Firtel, R. A.; Ginsberg M. H.; Borisy, G.; Parsons, J. T.; Horwitz, A. R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709.
du Roure, O.; Saez, A.; Buguin, A.; Austin, R. H.; Chavrier, P.; Siberzan, P.; Ladoux, B. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA 2005, 102, 2390–2395.
Beningo, K. A.; Dembo, M.; Kaverina, I.; Small, J. V.; Wang, Y. L. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 2001, 153, 881–888.
Sharp, D. J.; Brown, H. M.; Kwon, M.; Rogers, G. C.; Holland, G.; Scholey, J. M. Functional coordination of three mitotic motors in Drosophila embryos. Mol. Biol. Cell 2000, 11, 241–253.
Karsenti, E.; Vernos, I. The mitotic spindle: A self-made machine. Science 2001, 294, 543–547.
McIntosh, J. R.; Grishchuk, E. L.; West, R. R. Chromosome-microtubule interactions during mitosis. Annu. Rev. Cell Dev. Biol. 2002, 18, 193–219.
Dix, C. L.; Matthews, H. K.; Uroz, M.; McLaren, S.; Wolf, L.; Heatley, N.; Win, Z.; Almada, P.; Henriques, R.; Boutros, M. et al. The role of mitotic cell-substrate adhesion re-modeling in animal cell division. Dev. Cell 2018, 45, 132–145.e3.
Jones, M. C.; Askari, J. A.; Humphries, J. D.; Humphries, M. J. Cell adhesion is regulated by CDK1 during the cell cycle. J. Cell Biol. 2018, 217, 3203–3218.
Kunda, P.; Pelling, A. E.; Liu, T.; Baum, B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr. Biol. 2008, 18, 91–101.
Bergert, M.; Lendenmann, T.; Zündel, M.; Ehret, A. E.; Panozzo, D.; Richner, P.; Kim, D. K.; Kress, S. J. P.; Norris, D. J.; Sorkine-Hornung, O. et al. Confocal reference free traction force microscopy. Nat. Commun. 2016, 7, 12814.
Siegel, R. L.; Miller, K. D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30.
Jordan, M. A.; Toso, R. J.; Thrower, D.; Wilson, L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc. Natl. Acad. Sci. USA 1993, 90, 9552–9556.
Lock, J. G.; Jones, M. C.; Askari, J. A.; Gong, X. W.; Oddone, A.; Olofsson, H.; Göransson, S.; Lakadamyali, M.; Humphries, M. J.; Strömblad, S. Reticular adhesions are a distinct class of cell-matrix adhesions that mediate attachment during mitosis. Nat. Cell Biol. 2018, 20, 1290–1302.
Jordan, M. A.; Thrower, D.; Wilson, L. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J. Cell Sci. 1992, 102, 401–416.
Rizk, R. S.; Bohannon, K. P.; Wetzel, L. A.; Powers, J.; Shaw, S. L.; Walczak, C. E. MCAK and paclitaxel have differential effects on spindle microtubule organization and dynamics. Mol. Biol. Cell 2009, 20, 1639–1651.
Murrell, M. P.; Voituriez, R.; Joanny, J. F.; Nassoy, P.; Sykes, C.; Gardel, M. L. Liposome adhesion generates traction stress. Nat. Phys. 2014, 10, 163–169.
Wu, Y. L.; Engl, W.; Hu, B. H.; Cai, P. Q.; Leow, W. R.; Tan, N. S.; Lim, C. T.; Chen, X. D. Nanomechanically visualizing drug-cell interaction at the early stage of chemotherapy. ACS Nano 2017, 11, 6996–7005.
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (Nos. 11872355, 11627803, 12072339, and 11872354) and the Strategic Priority Research Program of the Chinese Academy of Science (No. XDB22040502).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, J., Su, Y. et al. Quantifying 3D cell-matrix interactions during mitosis and the effect of anticancer drugs on the interactions. Nano Res. 14, 4163–4172 (2021). https://doi.org/10.1007/s12274-021-3357-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3357-4