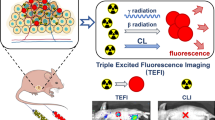
Abstract
Second near-infrared (NIR-II) fluorescence imaging is a recently emerged technique and is highly useful for accurate diagnosis of cancer. Although a diverse array of fluorescent nanomaterials have been developed to enable NIR-II fluorescence in various situations, they normally fail to unify the clinical techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI). Therefore, exploiting multimodal agents to integrate the newly emerged NIR-II fluorescence and traditional clinical techniques would be of key significance. Here, we report a rational fabrication of neodymium (Nd)-doped gadolinium tungstate nanoparticles (NPs) that are subsequentially decorated with a hydrophilic layer and demonstrate that they can achieve the harmonious integration of NIR-II fluorescence imaging, CT, and MRI. The NIR-II fluorescence emission was activated by an incident light with discrete wavelength ranging from 250 to 810 nm. NIR-II fluorescence-CT-MRI associated trimodal imaging was subsequently demonstrated for breast cancer by an 808 nm laser, along with the estimation of NIR-II fluorescence imaging for cervical cancer. The integration of newly emerged and traditional clinical imaging techniques highlights the huge potential of rare-earth-doped NPs for multimodal imaging of different types of cancer.

Similar content being viewed by others
References
Luciani, S.; Cabanes, A.; Prieto-Lara, E.; Gawryszewski, V. Cervical and female breast cancers in the Americas: Current situation and opportunities for action. Bull. World Health Organ. 2013, 91, 640–649.
Ginsburg, O.; Bray, F.; Coleman, M. P.; Vanderpuye, V.; Eniu, A.; Kotha, S. R.; Sarker, M.; Huong, T. T.; Allemani, C.; Dvaladze, A. et al. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017, 389, 847–860.
Torre, L. A.; Islami, F.; Siegel, R. L.; Ward, E. M.; Jemal, A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 444–457.
Tsu, V. D.; Jeronimo, J.; Anderson, B. O. Why the time is right to tackle breast and cervical cancer in low-resource settings. Bull. World Health Organ. 2013, 91, 683–690.
Liu, M. H.; Guo, H. B.; Liu, H. B.; Zhang, Z. Y.; Chi, C. W.; Hui, H.; Dong, D.; Hu, Z. H.; Tian, J. In vivo pentamodal tomographic imaging for small animals. Biomed. Opt. Express 2017, 8, 1356–1371.
Goel, S.; Ferreira, C. A.; Chen, F.; Ellison, P. A.; Siamof, C. M.; Barnhart, T. E.; Cai, W. B. Activatable hybrid nanotheranostics for tetramodal imaging and synergistic photothermal/photodynamic therapy. Adv. Mater. 2018, 30, 1704367.
Rieffel, J.; Chen, F.; Kim, J.; Chen, G. Y.; Shao, W.; Shao, S.; Chitgupi, U.; Hernandez, R.; Graves, S. A.; Nickles, R. J. et al. Hexamodal imaging with porphyrin-phospholipid-coated upconversion nanoparticles. Adv. Mater. 2015, 27, 1785–1790.
Miranda, D.; Carter, K.; Luo, D. D.; Shao, S.; Geng, J. M.; Li, C. N.; Chitgupi, U.; Turowski, S. G.; Li, N. S.; Atilla-Gokcumen, E. A. et al. Multifunctional liposomes for image-guided intratumoral chemo-phototherapy. Adv. Healthc. Mater. 2017, 6, 1700253.
Grodzinski, P.; Kircher, M.; Goldberg, M.; Gabizon, A. Integrating nanotechnology into cancer care. ACS Nano 2019, 13, 7370–7376.
Lusic, H.; Grinstaff, M. W. X-ray-computed tomography contrast agents. Chem. Rev. 2013, 113, 1641–1666.
Lee, N.; Yoo, D.; Ling, D. S.; Cho, M. H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2015, 115, 10637–10689.
Yankeelov, T. E.; Mankoff, D. A.; Schwartz, L. H.; Lieberman, F. S.; Buatti, J. M.; Mountz, J. M.; Erickson, B. J.; Fennessy, F. M. M.; Huang, W.; Kalpathy-Cramer, J. et al. Quantitative imaging in cancer clinical trials. Clin. Cancer Res. 2016, 22, 284–290.
O’Connor, J. P. B.; Aboagye, E. O.; Adams, J. E.; Aerts, H. J. W. L.; Barrington, S. F.; Beer, A. J.; Boellaard, R.; Bohndiek, S. E.; Brady, M.; Brown, G. et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2017, 14, 169–186.
Yu, X. J.; Li, A.; Zhao, C. Z.; Yang, K.; Chen, X. Y.; Li, W. W. Ultrasmall semimetal nanoparticles of bismuth for dual-modal computed tomography/photoacoustic imaging and synergistic thermoradiotherapy. ACS Nano 2017, 11, 3990–4001.
Frangioni, J. V. New technologies for human cancer imaging. J. Clin. Oncol. 2018, 26, 4012–4021.
Smith, A. M.; Mancini, M. C.; Nie, S. M. Second window for in vivo imaging. Nat. Nanotechnol. 2019, 4, 710–711.
Welsher, K.; Liu, Z.; Sherlock, S. P.; Robinson, J.; Chen, Z.; Daranciang, D.; Dai, H. J. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 2009, 4, 773–780.
Sun, C. X.; Li, B. H.; Zhao, M. Y.; Wang, S. F.; Lei, Z. H.; Lu, L. F.; Zhang, H. X.; Feng, L. S.; Dou, C. R.; Yin, D. R. et al. J-aggregates of cyanine dye for NIR-II in vivo dynamic vascular imaging beyond 1500 nm. J. Am. Chem. Soc. 2019, 141, 19221–19225.
Kang, Y. W.; Yu, X. J.; Fan, X. Y.; Aodenggerile, Zhao, S. Z.; Tu, C. L.; Yan, Z. Q.; Wang, R. B.; Li, W. W.; Qiu, H. B. Tetramodal imaging and synergistic cancer radio-chemotherapy enabled by multiple component-encapsulated zeolitic imidazolate frameworks. ACS Nano 2020, 14, 4336–4351.
Jiang, Y. Y.; Upputuri, P. K.; Xie, C.; Zeng, Z. L.; Sharma, A.; Zhen, X.; Li, J. C.; Huang, J. G.; Pramanik, M.; Pu, K. Y. Metabolizable semiconducting polymer nanoparticles for second near-infrared photoacoustic imaging. Adv. Mater. 2019, 31, 1808166.
He, S. Q.; Song, J.; Qu, J. L.; Cheng, Z. Crucial breakthrough of second near-infrared biological window fluorophores: Design and synthesis toward multimodal imaging and theranostics. Chem. Soc. Rev. 2018, 47, 4258–4278.
Kantamneni, H.; Zevon, M.; Donzanti, M. J.; Zhao, X. Y.; Sheng, Y.; Barkund, S. R.; McCabe, L. H.; Banach-Petrosky, W.; Higgins, L. M.; Ganesan, S. et al. Surveillance nanotechnology for multi-organ cancer metastases. Nat. Biomed. Eng. 2017, 1, 993–1003.
Gong, H.; Peng, R.; Liu, Z. Carbon nanotubes for biomedical imaging: The recent advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963.
Hong, G. S.; Diao, S.; Chang, J. L.; Antaris, A. L.; Chen, C. X.; Zhang, B.; Zhao, S.; Atochin, D. N.; Huang, P. L.; Andreasson, K. I. et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photonics 2014, 8, 723–730.
Hong, G. S.; Robinson, J. T.; Zhang, Y. J.; Diao, S.; Antaris, A. L.; Wang, Q. B.; Dai, H. J. In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew. Chem., Int. Ed. 2012, 51, 9818–9821.
Zhang, M. X.; Yue, J. Y.; Cui, R.; Ma, Z. R.; Wan, H.; Wang, F. F.; Zhu, S. J.; Zhou, Y.; Kuang, Y.; Zhong, Y. T. et al. Bright quantum dots emitting at ∼ 1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6590–6595.
Yang, T.; Tang, Y. A.; Liu, L.; Lv, X. Y.; Wang, Q. L.; Ke, H. T.; Deng, Y. B.; Yang, H.; Yang, X. L.; Liu, G. et al. Size-dependent Ag2S nanodots for second near-infrared fluorescence/photoacoustics imaging and simultaneous photothermal therapy. ACS Nano 2017, 11, 1848–1857.
Zhu, S. J.; Tian, R.; Antaris, A. L.; Chen, X. Y.; Dai, H. J. Near-infrared-II molecular dyes for cancer imaging and surgery. Adv. Mater. 2019, 31, 1900321.
Li, B. H.; Lu, L. F.; Zhao, M. Y.; Lei, Z. H.; Zhang, F. An efficient 1064 nm NIR-II excitation fluorescent molecular dye for deep-tissue high-resolution dynamic bioimaging. Angew. Chem., Int. Ed. 2018, 57, 7483–7487.
Antaris, A. L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G. S.; Qu, C. R.; Diao, S.; Deng, Z. X.; Hu, X. M.; Zhang, B. et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242.
Chen, C.; Ni, X.; Jia, S.; Liang, Y.; Wu, X.; Kong, D.; Ding, D. Massively evoking immunogenic cell death by focused mitochondrial oxidative stress using an AIE luminogen with a twisted molecular structure. Adv. Mater. 2019, 31, 1904914.
Chen, C.; Ou, H.; Liu, R.; Ding, D. Regulating the photophysical property of organic/polymer optical agents for promoted cancer phototheranostics. Adv. Mater. 2020, 32, 1806331.
Ni, X.; Zhang, X.; Duan, X.; Zheng, H. L.; Xue, X. S.; Ding, D. Near-infrared afterglow luminescent aggregation-Induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 2019, 19, 318–330.
Guo, B.; Chen, J. Q.; Chen, N. B.; Middha, E.; Xu, S. D.; Pan, Y. T.; Wu, M.; Li, K.; Liu, C. B.; Liu, B. High-resolution 3D NIR-II photoacoustic imaging of cerebral and tumor vasculatures using conjugated polymer nanoparticles as contrast agent. Adv. Mater. 2019, 31, 1808355.
Hong, G. S.; Zou, Y. P.; Antaris, A. L.; Diao, S.; Wu, D.; Cheng, K.; Zhang, X. D.; Chen, C. X.; Liu, B.; He, Y. H. et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat. Commun. 2014, 5, 4206.
Zhang, H. X.; Fan, Y.; Pei, P.; Sun, C. X.; Lu, L. F.; Zhang, F. Tm3+-sensitized NIR-II fluorescent nanocrystals for in vivo information storage and decoding. Angew. Chem., Int. Ed. 2019, 58, 10153–10157.
Wang, S. F.; Liu, L.; Fan, Y.; El-Toni, A. M.; Alhoshan, M. S.; Li, D. D.; Zhang, F. In vivo high-resolution ratiometric fluorescence imaging of inflammation using NIR-II nanoprobes with 1550 nm emission. Nano Lett. 2019, 19, 2418–2427.
Naczynski, D. J.; Tan, M. C.; Zevon, M.; Wall, B.; Kohl, J.; Kulesa, A.; Chen, S.; Roth, C. M.; Riman, R. E.; Moghe, P. V. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat. Commun. 2013, 4, 2199.
Chen, G. Y.; Ohulchanskyy, T. Y.; Liu, S.; Law, W. C.; Wu, F.; Swihart, M. T.; Ågren, H.; Prasad, P. N. Core/shell NaGdF4: Nd3+/NaGdF4 nanocrystals with efficient near-infrared to near-infrared downconversion photoluminescence for bioimaging applications. ACS Nano 2012, 6, 2969–2977.
Lammers, T.; Ferrari, M. The success of nanomedicine. Nano Today 2020, 31, 100853.
D’Mello, S. R.; Cruz, C. N.; Chen, M. L.; Kapoor, M.; Lee, S. L.; Tyner, K. M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017, 12, 523–529.
Bobo, D.; Robinson, K. J.; Islam, J.; Thurecht, K. J.; Corrie, S. R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387.
Bonvalot, S.; Rutkowski, P. L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M. P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M. et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): A multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159.
Wang, R.; Li, X. M.; Zhou, L.; Zhang, F. Epitaxial seeded growth of rare-earth nanocrystals with efficient 800 nm near-infrared to 1525 nm short-wavelength infrared downconversion photoluminescence for in vivo bioimaging. Angew. Chem., Int. Ed. 2014, 53, 12086–12090.
Naczynski, D. J.; Sun, C.; Türkcan, S.; Jenkins, C.; Koh, A. L.; Ikeda, D.; Pratx, G.; Xing, L. X-ray-induced shortwave infrared biomedical imaging using rare-earth nanoprobes. Nano Lett. 2015, 15, 96–102.
Dang, X. N.; Gu, L.; Qi, J. F.; Correa, S.; Zhang, G. R.; Belcher, A. M.; Hammond, P. T. Layer-by-layer assembled fluorescent probes in the second near-infrared window for systemic delivery and detection of ovarian cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 5179–5184.
Zhong, Y. T.; Ma, Z. R.; Zhu, S. J.; Yue, J. Y.; Zhang, M. X.; Antaris, A. L.; Yuan, J.; Cui, R.; Wan, H.; Zhou, Y. et al. Boosting the downshifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat. Commun. 2017, 8, 737.
Ding, F.; Zhan, Y. B.; Lu, X. J.; Sun, Y. Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging. Chem. Sci. 2018, 9, 4370–4380.
Yu, X. J.; Liu, X. Y.; Wu, W. J.; Yang, K.; Mao, R. H.; Ahmad, F.; Chen, X. Y.; Li, W. W. CT/MRI-guided synergistic radiotherapy and X-ray inducible photodynamic therapy using Tb-doped Gd-W-nanoscintillators. Angew. Chem., Int. Ed. 2019, 58, 2017–2022.
Guo T.; Lin, Y.; Zhang, W. J.; Hong, J. S.; Lin, R. H.; Wu, X. P.; Li, J.; Lu, C. H.; Yang, H. H. High-efficiency X-ray luminescence in Eu3+-activated tungstate nanoprobes for optical imaging through energy transfer sensitization. Nanoscale 2018, 10, 1607–1612.
Hudson, D. E.; Hudson, D. O.; Wininger, J. M.; Richardson, B. D. Penetration of laser light at 808 and 980 nm in bovine tissue samples. Photomed. Laser Surg. 2013, 31, 163–168.
Lyu, Y.; Xie, C.; Chechetka, S. A.; Miyako, E.; Pu, K. Y. Semiconducting polymer nanobioconjugates for targeted photothermal activation of neurons. J. Am. Chem. Soc. 2016, 138, 9049–9052.
Zhong, Y. T.; Tian, G.; Gu, Z. J.; Yang, Y. J.; Gu, L.; Zhao, Y. L.; Ma, Y.; Yao, J. N. Elimination of photon quenching by a transition layer to fabricate a quenching-shield sandwich structure for 800 nm excited upconversion luminescence of Nd3+-sensitized nanoparticles. Adv. Mater. 2014, 26, 2831–2837.
Kotagiri, N.; Sudlow, G. P.; Akers, W. J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379.
Chen, Q.; Wu, J.; Ou, X.; Huang, B.; Almutlaq, J.; Zhumekenov, A.; Guan, X.; Han, S.; Liang, L.; Yi, Z. et. al. All-inorganic perovskite nanocrystal scintillators. Nature 2018, 561, 88–93.
Nashed, R.; Alamgir, F. M.; Jang, S. S.; Ismail, Y.; El-Sayed, M. A.; Allam, N. K. Bandgap bowing in Ta-W-O system for efficient solar energy conversion: Insights from density functional theory and X-ray diffraction. Appl. Phys. Lett. 2013, 103, 133905.
Ye, S.; Song, E. H.; Zhang, Q. Y. Transition metal-involved photon upconversion. Adv. Sci. 2016, 3, 1600302.
Pernodet, N.; Fang, X. H.; Sun, Y.; Bakhtina, A.; Ramakrishnan, A.; Sokolov, J.; Ulman, A.; Rafailovich, M. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small 2006, 2, 766–773.
Li, M. H.; Sun, X. T.; Zhang, N.; Wang, W.; Yang, Y.; Jia, H. Z.; Liu, W. G. NIR-activated polydopamine-coated carrier-free “nanobomb” for in situ on-demand drug release. Adv. Sci. 2018, 5, 1800155.
Zhou, J. J.; Xiong, Q. R.; Ma, J. L.; Ren, J. H.; Messersmith, P. B.; Chen, P.; Duan, H. W. Polydopamine-enabled approach toward tailored plasmonic nanogapped nanoparticles: From nanogap engineering to multifunctionality. ACS Nano 2016, 10, 11066–11075.
Choi, C. K. K.; Chiu, Y. T. E.; Zhuo, X. L.; Liu, Y.; Pak, C. Y.; Liu, X. D.; Tse, Y. L. S.; Wang, J. F.; Choi, C. H. J. Dopamine-mediated assembly of citrate-capped plasmonic nanoparticles into stable core-shell nanoworms for intracellular applications. ACS Nano 2019, 13, 5864–5884.
Swierczewska, M.; Cho, K. Y.; Mertz, E. L.; Huang, X. L.; Zhang, F.; Zhu, L.; Yoon, H. Y.; Park, J. H.; Bhirde, A.; Lee, S. et al. A facile, one-step nanocarbon functionalization for biomedical applications. Nano Lett. 2012, 12, 3613–3620.
Zhou, J.; Li, M. H.; Hou, Y. H.; Luo, Z.; Chen, Q. F.; Cao, H. X.; Huo, R. L.; Xue, C. C.; Sutrisno, L.; Hao, L. et al. Engineering of a nanosized biocatalyst for combined tumor starvation and low-temperature photothermal therapy. ACS Nano 2018, 12, 2858–2872.
Kim, S.; Jang, Y.; Jang, L. K.; Sunwoo, S. H.; Kim, T. I.; Cho, S. W.; Lee, J. Y. Electrochemical deposition of dopamine-hyaluronic acid conjugates for anti-biofouling bioelectrodes. J. Mater. Chem. B 2017, 5, 4507–4513.
Nyk, M.; Kumar, R.; Ohulchanskyy, T. Y.; Bergey, E. J.; Prasad, P. N. High contrast in vitro and in vivo photoluminescence bioimaging using near infrared to near infrared up-conversion in Tm3+ and Yb3+ doped fluoride nanophosphors. Nano Lett. 2008, 8, 3834–3838.
Rocha, U.; Kumar, K. U.; Jacinto, C.; Villa, I.; Sanz-Rodríguez, F.; del Carmen Iglesias de la Cruz M.; Juarranz, A.; Carrasco, E.; van Veggel, F. C. J. M.; Bovero, E.; Solé, J. G. et al. Neodymium-doped LaF3 nanoparticles for fluorescence bioimaging in the second biological window. Small 2014, 10, 1141–1154.
Heffern, M. C.; Matosziuk, L. M.; Meade, T. J. Lanthanide probes for bioresponsive imaging. Chem. Rev. 2014, 114, 4496–4539.
Kohane, D. S.; Langer, R. Biocompatibility and drug delivery systems. Chem. Sci. 2010, 1, 441–446.
Rieffel, J.; Chitgupi, U.; Lovell, J. F. Recent advances in higherorder, multimodal, biomedical imaging agents. Small 2015, 11, 4445–4461.
Hyafil, F.; Cornily, J. C.; Feig, J. E.; Gordon, R.; Vucic, E.; Amirbekian, V.; Fisher, E. A.; Fuster, V.; Feldman, L. J.; Fayad, Z. A. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 2007, 13, 636–641.
Cho, N. H.; Cheong, T. C.; Min, J. H.; Wu, J. H.; Lee, S. J.; Kim, D.; Yang, J. S.; Kim, S.; Kim, Y. K.; Seong, S. Y. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat. Nanotechnol. 2011, 6, 675–682.
Wang, X.; Guo, Z.; Zhang, C. Y.; Zhu, S.; Li, L. L.; Gu, Z. J.; Zhao, Y. L. Ultrasmall BiOI quantum dots with efficient renal clearance for enhanced radiotherapy of cancer. Adv. Sci. 2020, 7, 1902561.
Chen, X. F.; Song, J. B.; Chen, X. Y.; Yang, H. H. X-ray-activated nanosystems for theranostic applications. Chem. Soc. Rev. 2019, 48, 3073–3101.
Ni, D. L.; Zhang, J. W.; Wang, J.; Hu, P.; Jin, Y. Y.; Tang, Z. M.; Yao, Z. W.; Bu, W. B.; Shi, J. L. Oxygen vacancy enables markedly enhanced magnetic resonance imaging-guided photothermal therapy of a Gd3+-doped contrast agent. ACS Nano 2017, 11, 4256–4264.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 81901885), the Science and Technology Commission of Shanghai Municipality (Nos. 17JC1400700, 18JC1415500, and 1952710400), the Shanghai Education Development Foundation and the Shanghai Municipal Education Commission (No. 16SG54), and the Cultivating Fund of Frontiers Science Center for Transformative Molecules (No. 2019PT02). The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2020_3136_MOESM1_ESM.pdf
Integrating the second near-infrared fluorescence imaging with clinical techniques for multimodal cancer imaging by neodymium-doped gadolinium tungstate nanoparticles
Rights and permissions
About this article
Cite this article
Yu, X., Aodenggerile, Jiang, Z. et al. Integrating the second near-infrared fluorescence imaging with clinical techniques for multimodal cancer imaging by neodymium-doped gadolinium tungstate nanoparticles. Nano Res. 14, 2160–2170 (2021). https://doi.org/10.1007/s12274-020-3136-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-3136-7