Abstract
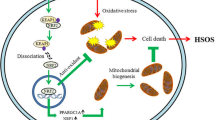
Pyrrolizidine alkaloids (PAs) are potent hepatotoxins that can cause liver damage. Hyperoside (Hyp), a natural flavonoid, can be extracted from medicinal plants. Hyp displays hepatoprotective activity in various liver diseases. However, the potential effect and mechanism of action of Hyp in ameliorating PA-induced liver injury remain obscure. This study aimed to explore the protective effect of Hyp against PA-induced hepatotoxicity and its underlying mechanism. We established an in vitro model of PAs in mouse primary hepatocytes and developed a mouse model of acute PA toxicity to investigate the protective effect of Hyp. We found that Hyp notably attenuated PA-induced hepatotoxicity. RNA-sequencing showed that the beneficial effect of Hyp against PA-induced hepatotoxicity was associated with the transcription factor EB (TFEB)-peroxisome proliferator-activated receptor-γ coactivator-1-α (PGC1α) pathway. Our results confirmed that both the autophagy-lysosomal pathway and mitochondrial biogenesis were induced by Hyp through TFEB nuclear translocation in PA-induced liver injury. Furthermore, we demonstrated that activation of the mechanistic target of rapamycin complex 1 (mTORC1) by MHY 1485 decreased TFEB nuclear translocation and abrogated the protective effect of Hyp against PA-induced liver injury in mice. In contrast, inhibition of mTORC1 activity increased the level of TFEB and reduced hepatotoxicity induced by PAs in mouse livers. Likewise, Hyp-induced TFEB activation was validated in vitro. In conclusion, Hyp can activate the TFEB-mediated autophagy-lysosomal pathway and mitochondrial biogenesis through inhibition of mTORC1 activity, alleviating the liver injury induced by PAs, thus suggesting the potential value of Hyp in the treatment of PA-induced hepatotoxicity.








Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available due to resasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy RK (2021) ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 116:878–898. https://doi.org/10.14309/ajg.0000000000001259
Chao X, Wang S, Zhao K, Li Y, Williams JA, Li T, Chavan H, Krishnamurthy P, He XC, Li L, Ballabio A, Ni HM, Ding W (2018) Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology 155:865–879. https://doi.org/10.1053/j.gastro.2018.05.027
Chao X, Wang S, Yang L, Ni HM, Ding W (2021) Trehalose activates hepatic transcription factor EB (TFEB) but fails to ameliorate alcohol-impaired TFEB and liver injury in mice. Alcohol Clin Exp Res 45:1950–1964. https://doi.org/10.1111/acer.14695
Eskelinen EL (2006) Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med 27:495–502. https://doi.org/10.1016/j.mam.2006.08.005
Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ, Liu ZX, Kaplowitz N (2013) Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol Sci 34:243–253. https://doi.org/10.1016/j.tips.2013.01.009
Hu C, Chen Y, Cao Y, Jia Y, Zhang J (2020) Metabolomics analysis reveals the protective effect of quercetin-3-O-galactoside (Hyperoside) on liver injury in mice induced by acetaminophen. J Food Biochem 3:e13420. https://doi.org/10.1111/jfbc.13420
Huang Z, Jing X, Sheng Y, Zhang J, Hao Z, Wang Z, Ji L (2019) (-)-Epicatechin attenuates hepatic sinusoidal obstruction syndrome by inhibiting liver oxidative and inflammatory injury. Redox Biol 22:101117. https://doi.org/10.1016/j.redox.2019.101117
Hunt CM (2010) Mitochondrial and immunoallergic injury increase risk of positive drug rechallenge after drug-induced liver injury: a systematic review. Hepatology 52:2216–2222. https://doi.org/10.1002/hep.24022
Kaplowitz N (2005) Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov 4:489–499. https://doi.org/10.1038/nrd1750
Leise MD, Poterucha JJ, Talwalkar JA (2014) Drug-induced liver injury. Mayo Clin Proc 89:95–106. https://doi.org/10.1016/j.mayocp.2013.09.016
Liesa M, Palacin M, Zorzano A (2009) Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89:799–845. https://doi.org/10.1152/physrev.00030.2008
Liu W, Ye L, Huang W, Guo L, Xu Z, Wu H, Yang C, Liu H (2016) p62 links the autophagy pathway and the ubiquitin-proteasome system upon ubiquitinated protein degradation. Cell Mol Biol Lett 21:29. https://doi.org/10.1186/s11658-016-0031-z
Lu Y, Ma J, Song Z, Ye Y, Fu PP, Lin G (2018) The role of formation of pyrrole-ATP synthase subunit beta adduct in pyrrolizidine alkaloid-induced hepatotoxicity. Arch Toxicol 92:3403–3414. https://doi.org/10.1007/s00204-018-2309-6
Ma X, Liu H, Murphy JT, Foyil SR, Godar RJ, Abuirqeba H, Weinheimer CJ, Barger PM, Diwan A (2015) Regulation of the transcription factor EB-PGC1alpha axis by beclin-1 controls mitochondrial quality and cardiomyocyte death under stress. Mol Cell Biol 35:956–976. https://doi.org/10.1128/MCB.01091-14
Mansouri A, Gattolliat CH, Asselah T (2018) Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 155:629–647. https://doi.org/10.1053/j.gastro.2018.06.083
Martina JA, Puertollano R (2013) Rag GTPases mediate amino acid-dependent recruitment of TFEB and MITF to lysosomes. J Cell Biol 200:475–491. https://doi.org/10.1083/jcb.201209135
Martina JA, Chen Y, Gucek M, Puertollano R (2012) MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8:903–914. https://doi.org/10.4161/auto.19653
Middleton E, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741. https://doi.org/10.1016/j.cell.2011.10.026
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326. https://doi.org/10.1016/j.cell.2010.01.028
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13. https://doi.org/10.1042/BJ20081386
Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y (2009) Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 10:458–467. https://doi.org/10.1038/nrm2708
Navarro VJ, Senior JR (2006) Drug-related hepatotoxicity. N Engl J Med 354:731–739. https://doi.org/10.1056/NEJMra052270
Neuman MG, Cohen L, Opris M, Nanau RM, Hyunjin J (2015) Hepatotoxicity of pyrrolizidine alkaloids. J Pharm Pharm Sci 18:825–843. https://doi.org/10.18433/j3bg7j
Niu C, Ma M, Han X, Wang Z, Li H (2017) Hyperin protects against cisplatin-induced liver injury in mice. Acta Cir Bras 32:633–640. https://doi.org/10.1590/s0102-865020170080000005
Pastore N, Vainshtein A, Klisch TJ, Armani A, Huynh T, Herz NJ, Polishchuk EV, Sandri M, Ballabio A (2017) TFE3 regulates whole-body energy metabolism in cooperation with TFEB. EMBO Mol Med 9:605–621. https://doi.org/10.15252/emmm.201607204
Pessayre D, Fromenty B, Berson A, Robin MA, Lettéron P, Moreau R, Mansouri A (2012) Central role of mitochondria in drug-induced liver injury. Drug Metab Rev 44:34–87. https://doi.org/10.3109/03602532.2011.604086
Puertollano R, Ferguson SM, Brugarolas J, Ballabio A (2018) The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J 37:e98804. https://doi.org/10.15252/embj.201798804
Qian H, Chao X, Williams J, Fulte S, Li T, Yang L, Ding W (2021) Autophagy in liver diseases: a review. Mol Aspects Med 82:100973. https://doi.org/10.1016/j.mam.2021.100973
Raben N, Puertollano R (2016) TFEB and TFE3: linking lysosomes to cellular adaptation to stress. Annu Rev Cell Dev Biol 32:255–278. https://doi.org/10.1146/annurev-cellbio-111315-125407
Raza A, Xu X, Sun H, Tang J, Ouyang Z (2017) Pharmacological activities and pharmacokinetic study of hyperoside: a short review. Trop J Pharma Res 16:483–489. https://doi.org/10.4314/tjpr.v16i2.30
Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM (2012) The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal 5:ra42. https://doi.org/10.1126/scisignal.2002790
Ruivo R, Anne C, Sagne C, Gasnier B (2009) Molecular and cellular basis of lysosomal transmembrane protein dysfunction. Biochim Biophys Acta 1793:636–649. https://doi.org/10.1016/j.bbamcr.2008.12.008
Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A (2009) A gene network regulating lysosomal biogenesis and function. Science 325:473–477. https://doi.org/10.1126/science.1174447
Schwake M, Schroder B, Saftig P (2013) Lysosomal membrane proteins and their central role in physiology. Traffic 14:739–748. https://doi.org/10.1111/tra.12056
Settembre C, Malta CD, Polito VA, Arencibia MG, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A (2011) TFEB links autophagy to lysosomal biogenesis. Science 332:1429–1433. https://doi.org/10.1126/science.1204592
Settembre C, Cegli RD, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Klisch TJ, Wollenberg AC, Bernardo DD, Chan L, Irazoqui JE, Ballabio A (2013) TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol 15:647–658. https://doi.org/10.1038/ncb2718
Settembre C, Fraldi A, Medina DL, Ballabio A (2013) Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol 14:283–296. https://doi.org/10.1038/nrm3565
Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, Xu J, Niu J, Liu J, Watkins PB, Aithal GP, Andrade RJ, Dou X, Yao L, Lv F, Wang Q, Li Y, Zhou X, Zhang Y, Zong P, Wan B, Zou Z, Yang D, Nie Y, Li D, Wang Y, Han X, Zhuang H, Mao Y, Chen C (2019) Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology 156:2230–2241. https://doi.org/10.1053/j.gastro.2019.02.002
Stegelmeier BL, Edgar JA, Colegate SM, Gardner DR, Schoch TK, Coulombe RA, Molyneux RJ (1999) Pyrrolizidine alkaloids plants, metabolism and toxicity. J Nat Toxins 8:95–116
Wang J, Gao H (2014) Tusanqi and hepatic sinusoidal obstruction syndrome. J Dig Dis 15:105–107. https://doi.org/10.1111/1751-2980.12112
Wang Y, Qiao D, Li Y, Xu F (2018) Risk factors for hepatic veno-occlusive disease caused by Gynura segetum: a retrospective study. BMC Gastroenterol 18:156. https://doi.org/10.1186/s12876-018-0879-7
Wang W, Yang X, Chen Y, Ye X, Jiang K, Xiong A, Yang L, Wang Z (2020) Seneciphylline, a main pyrrolizidine alkaloid in Gynura japonica, induces hepatotoxicity in mice and primary hepatocytes via activating mitochondria-mediated apoptosis. J Appl Toxicol 40:1534–1544. https://doi.org/10.1002/jat.4004
Xie W, Jiang Z, Wang J, Zhang X, Melzig MF (2016) Protective effect of hyperoside against acetaminophen (APAP) induced liver injury through enhancement of APAP clearance. Chem Biol Interact 246:11–19. https://doi.org/10.1016/j.cbi.2016.01.004
Xing H, Fu R, Cheng C, Cai Y, Wang X, Deng D, Gong X, Chen J (2020) Hyperoside protected against oxidative stress-induced liver injury via the PHLPP2-AKT-GSK-3β signaling pathway in vivo and in vitro. Front Pharmacol 11:1065–1077. https://doi.org/10.3389/fphar.2020.01065
Xiong A, Shao Y, Fang L, Yang X, Zhang S, Zheng J, Ding W, Yang L, Wang Z (2019) Comparative analysis of toxic components in different medicinal parts of Gynura japonica and its toxicity assessment on mice. Phytomedicine 54:77–88. https://doi.org/10.1016/j.phymed.2018.06.015
Yan M, Huo Y, Yin S, Hu H (2018) Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol 17:274–283. https://doi.org/10.1016/j.redox.2018.04.019
Yang X, Wang H, Ni HM, Xiong A, Wang Z, Sesaki H, Ding W, Yang L (2017) Inhibition of Drp1 protects against senecionine-induced mitochondria-mediated apoptosis in primary hepatocytes and in mice. Redox Biol 12:264–273. https://doi.org/10.1016/j.redox.2017.02.020
Zheng P, Xu Y, Ren Z, Wang Z, Wang S, Xiong J, Zhang H, Jiang H (2021) Toxic prediction of pyrrolizidine alkaloids and structure-dependent induction of apoptosis in HepaRG cells. Oxid Med Cell Longev 2021:1–12. https://doi.org/10.1155/2021/8822304
Zhu X, Ji M, Han Y, Guo Y, Zhu W, Gao F, Yang X, Zhang C (2017) PGRMC1-dependent autophagy by hyperoside induces apoptosis and sensitizes ovarian cancer cells to cisplatin treatment. Int J Oncol 50:835–846. https://doi.org/10.3892/ijo.2017.3873
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 81920108033; 82074011; 82130115) and the Program of Shanghai Municipal Health Commission (No. ZY (2021–2023)-0215).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12272_2023_1460_MOESM2_ESM.tif
Supplementary file2 Supplementary Fig. 1 Hyp attenuates PA-induced liver injury by enhancing autophagy in primary hepatocytes. A Primary hepatocytes were treated with TA for 12 h or 24 h. Hyp was added to the cultures 1 h before TA treatment. The expression of the proteins LC3-II and SQSTM1/p62 were measured by western blotting in primary hepatocytes. Bar graphs show summary data. B Primary hepatocytes were treated with TA and Hyp in the presence or absence of Baf A1. The expression of the protein LC3-II was measured by western blotting in primary hepatocytes. C Primary hepatocytes were transfected with Ad-mRFP-GFP-LC3 for 24 h. After transfection, cells were treated with TA for 12 h or 24 h, Hyp was added to the cultures 1 h before TA treatment. Representative images of Ad-mRFP-GFP-LC3 in primary hepatocytes. Scale bars, 200 μm. B-C Hyp, 50 μM. Data were shown as the means ± SD from at least three independent experiments and analyzed by one-way ANOVA. ###P < 0.001 vs. Vehicle; *P < 0.05, **P < 0.01, ***P < 0.001 vs. TA. (TIF 4416 KB)
12272_2023_1460_MOESM3_ESM.tif
Supplementary file3 Supplementary Fig. 2 Hyp attenuates PA-induced impairment of lysosomal function. A The expression of the protein LAMP1 was measured by western blotting in primary hepatocytes. Bar graphs show summary data. B The expression of the protein LAMP1 was measured by western blotting in mouse liver. Bar graphs show summary data (n = 3). B Hyp, 40 mg/kg. A Data are shown as the means ± SD from at least three independent experiments and analyzed by one-way ANOVA. B Data were shown as the means ± SEM and analyzed by one-way ANOVA. ###P < 0.001 vs. Vehicle; *P < 0.05, **P < 0.01, ***P < 0.001vs. TA. (TIF 1795 KB)
12272_2023_1460_MOESM4_ESM.tif
Supplementary file4 Supplementary Fig. 3 Hyp induces mitochondrial biogenesis through the TFEB-PGC1α pathway in PA-treated primary hepatocytes. A Total intracellular ATP level. B ΔΨm determined by JC-1 red/green fluorescence ratio. C Representative images of the mitochondria of primary hepatocytes stained with MitoTracker Green. D Primary hepatocytes were fractionated into mitochondria and cytosol, and the level of Cyt C was analyzed by western blotting. Fraction quality was verified by immunoblotting with markers for the mitochondria (COX IV) and cytoplasm (α-Tubulin). E Representative TEM micrographs showing Hyp ameliorates mitochondria damage in PAs-treated primary hepatocytes. Scale bars, 2 μm (wireframe indicates the magnified image). F The expression of the protein PGC1α was measured by western blotting in primary hepatocytes. Bar graphs show summary data. G Primary hepatocytes were transfected with scRNA or siTFEB for 24 h and treated with TA in the presence or absence of Hyp for 12 h or 24 h. The expression of the proteins TFEB and PGC1α were measured by western blotting. H The expression of the proteins CPT1α and PPARα were measured by western blotting in primary hepatocytes. Bar graphs show summary data. A-E, G, Hyp, 50 μM. Data were shown as the means ± SD from at least three independent experiments and analyzed by one-way ANOVA. ###P < 0.001 vs. Vehicle; *P < 0.05, **P < 0.01, ***P < 0.001 vs. TA. (TIF 2214 KB)
12272_2023_1460_MOESM5_ESM.tif
Supplementary file5 Supplementary Fig. 4 TFEB mediates the beneficial effects of Hyp in PA-induced liver injury. The mRNA levels of TFEB-associated genes (Map1lc3b, Uvrag, Wipi1, Lamp1, Pgc1α, Nrf1, Tfam) were evaluated by qRT-PCR. Data were shown as the means ± SEM and analyzed by one-way ANOVA or Student's t-test. ***P < 0.001 vs. TA; &&P < 0.01, &&&P < 0.001 vs. TA-Hyp (siRNA NC). (TIF 2275 KB)
12272_2023_1460_MOESM6_ESM.tif
Supplementary file6 Supplementary Fig. 5 Primary hepatocytes were transfected with a plasmid carrying TFEB or empty vector and then treated to 50 μM Hyp for 12 h or 24 h. Effects of Hyp on TFEB nuclear translocation in GFP-TFEB primary hepatocytes. Scale bars, 200 μm. Data were representative of at least three independent experiments. (TIF 1608 KB)
12272_2023_1460_MOESM7_ESM.tif
Supplementary file7 Supplementary Fig. 6 Inhibition of mTOR1 activity by Hyp attenuates PA-induced liver injury in primary hepatocytes. A The expression of the proteins p-mTORC and p-ERK1/2 were measured by western blotting in primary hepatocytes. Bar graphs show summary data. B The expression of the proteins p-S6 and p-eIF4E were measured by western blotting in primary hepatocytes. Bar graphs show summary data. C Primary hepatocytes treated with TA in the presence or absence of Torin1 for 12 h or 24 h. The expression of the nuclear and cytosolic TFEB proteins in primary hepatocytes were analyzed by western blotting. D Primary hepatocytes treated with TA and Hyp in the presence or absence of MHY 1485 for 12 h or 24 h. The expression of the nuclear and cytosolic TFEB proteins in primary hepatocytes were analyzed by western blotting. Data were shown as the means ± SD from at least three independent experiments and analyzed by one-way ANOVA. ##P < 0.01, ###P < 0.001 vs. Vehicle; *P < 0.05, **P < 0.01, ***P < 0.001 vs. TA. (TIF 4846 KB)
12272_2023_1460_MOESM8_ESM.tif
Supplementary file8 Supplementary Fig. 7 Torin1 attenuates PA-induced liver injury in mice. The mice were orally administered TA, and then were intraperitoneally injected with Torin1 once at 6 h after TA administration. A Serum ALT activity (n = 6). B Serum AST activity (n = 6). C Serum TBA amount (n = 6). D Representative images of H&E-stained liver sections (scale bars, 50 μm). E Nuclear fractions of TFEB in mouse liver was analyzed by western blotting (n = 3). F The mRNA levels of TFEB target genes (Map1lc3b, Uvrag, Wipi1, Lamp1, Pgc1α) were evaluated by qRT-PCR (n = 6). G Paraffin sections of mouse liver were stained with TFEB (red) and DAPI (blue). Scale bars, 50 μm (wireframe indicates the magnified image). Data were shown as the means ± SEM and analyzed by one-way ANOVA. A-C ***P < 0.001 vs. TA. F ###P < 0.001 vs. Vehicle; **P < 0.01, ***P < 0.001 vs. TA. (TIF 6776 KB)
12272_2023_1460_MOESM9_ESM.tif
Supplementary file9 Supplementary Fig. 8 Inhibition of mTORC1 activity by Hyp attenuates PA-induced liver injury in mice. Paraffin sections of mouse liver were stained with TFEB (red) and DAPI (blue). Scale bars, 50 μm (wireframe indicates the magnified image). (TIF 3939 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Xiong, A., Wang, X. et al. Hyperoside attenuates pyrrolizidine alkaloids-induced liver injury by ameliorating TFEB-mediated mitochondrial dysfunction. Arch. Pharm. Res. 46, 694–712 (2023). https://doi.org/10.1007/s12272-023-01460-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-023-01460-3