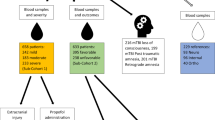
Abstract
Cardiac surgery with extracorporeal circulation is considered to be one of the surgical types with the highest incidence of delayed neurocognitive recovery (DNR), but the mechanism is unclear. Metabolomics technology can be used to understand the early postoperative metabolic profile and find the relationship between serum metabolites and disease. We performed untargeted analyses of postoperative serum metabolites in all surgical groups, as well as serum metabolites in healthy nonsurgical adults, by using liquid chromatography‒mass spectrometry (LC‒MS). DNR after cardiopulmonary bypass surgery occurred in 35% of surgical patients. Sixty-nine metabolites were found to be associated with DNR. Lipids and lipid-like molecules occupy a total of 55 positions. Lipid metabolism occupies an important position in the serum metabolic profile of DNR patients in the early postoperative period. Phosphatidylinositol (PI), sphingomyelin (SM), and phosphatidylglycerol (PG) appear at the highest frequency. Correlation analysis and receiver operator characteristic curve analysis confirmed PI and SM as potential biomarkers for an increased risk of DNR.
Graphical abstract






Similar content being viewed by others
References
Evered L, Silbert B, Knopman DS, Scott DA, Dekosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery—2018. Br J Anaesth. 2018;121(5):1005–12. https://doi.org/10.1016/j.bja.2017.11.087.
Hogue CW, Grafman J. Aligning nomenclature for cognitive changes associated with anaesthesia and surgery with broader diagnostic classifications of non-surgical populations: a needed first step. Br J Anaesth. 2018;121(5):991–3. https://doi.org/10.1016/j.bja.2017.12.029.
Bhamidipati D, Goldhammer JE, Sperling MR, Torjman MC, McCarey MM, Whellan DJ. Cognitive outcomes after coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2017;31(2):707–18. https://doi.org/10.1053/j.jvca.2016.09.028.
Zhang X, Lyu Y, Wang D. S100β as a potential biomarker of incident delirium: a systematic review and meta-analysis. Minerva Anestesiol. 2020;86(8):853–60. https://doi.org/10.23736/s0375-9393.20.14100-2.
Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. 2019;128(4):781–8. https://doi.org/10.1213/ane.0000000000004053.
Chi YL, Li ZS, Lin CS, Wang Q, Zhou YK. Evaluation of the postoperative cognitive dysfunction in elderly patients with general anesthesia. Eur Rev Med Pharmacol Sci. 2017;21(6):1346–54.
Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, McLean JA. Untargeted metabolomics strategies-challenges and emerging directions. J Am Soc Mass Spectrom. 2016;27(12):1897–905. https://doi.org/10.1007/s13361-016-1469-y.
Fiehn O. Metabolomics by gas chromatography-mass spectrometry: combined targeted and untargeted profiling. Curr Protoc Mol Biol. 2016;114:30.4.1-4.2. https://doi.org/10.1002/0471142727.mb3004s114.
Toledo JB, Arnold M, Kastenmüller G, Chang R, Baillie RA, Han X, et al. Metabolic network failures in Alzheimer’s disease: a biochemical road map. Alzheimers Dement. 2017;13(9):965–84. https://doi.org/10.1016/j.jalz.2017.01.020.
Luan H, Wang X, Cai Z. Mass spectrometry-based metabolomics: targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom Rev. 2019;38(1):22–33. https://doi.org/10.1002/mas.21553.
Shaefi S, Shankar P, Mueller AL, O’Gara BP, Spear K, Khabbaz KR, et al. Intraoperative oxygen concentration and neurocognition after cardiac surgery. Anesthesiology. 2021;134(2):189–201. https://doi.org/10.1097/aln.0000000000003650.
Wang F, Zou ZR, Yuan D, Gong Y, Zhang L, Chen X, et al. Correlation between serum S100β protein levels and cognitive dysfunction in patients with cerebral small vessel disease: a case-control study. Biosci Rep. 2017;37(2). 10.1042/bsr20160446
Zhang Y, Duan B, Wang L, Ye Z, Pan Y, Guo Q, et al. Association between the variability of cerebral oxygen saturation during cardiopulmonary bypass and delayed postoperative neurocognitive recovery in cardiac valve surgical patients: A pilot study. Int J Clin Pract. 2021;75(1):e13651. https://doi.org/10.1111/ijcp.13651.
Hu J, Li CJ, Wang BJ, Li XY, Mu DL, Wang DX. The sensitivity and specificity of statistical rules for diagnosing delayed neurocognitive recovery with Montreal cognitive assessment in elderly surgical patients: a cohort study. Medicine (Baltimore). 2020;99(29):e21193. https://doi.org/10.1097/md.0000000000021193.
Jiang Z, Cai Y, Zhang X, Lv Y, Zhang M, Li S, et al. Predicting delayed neurocognitive recovery after non-cardiac surgery using resting-state brain network patterns combined with machine learning. Front Aging Neurosci. 2021;13:715517. https://doi.org/10.3389/fnagi.2021.715517.
Lewis C, Levine A, Balmert LC, Chen L, Sherwani SS, Nemeth AJ, et al. Neurocognitive, quality of life, and behavioral outcomes for patients with covert stroke after cardiac surgery: exploratory analysis of data from a prospectively randomized trial. Anesth Analg. 2021;133(5):1187–96. https://doi.org/10.1213/ane.0000000000005690.
Bukauskienė R, Širvinskas E, Lenkutis T, Benetis R, Steponavičiūtė R. Risk factors for delayed neurocognitive recovery according to brain biomarkers and cerebral blood flow velocity. Medicina (Kaunas). 2020;56(6). https://doi.org/10.3390/medicina56060288
Danielson M, Wiklund A, Granath F, Blennow K, Mkrtchian S, Nellgård B, et al. Neuroinflammatory markers associate with cognitive decline after major surgery: findings of an explorative study. Ann Neurol. 2020;87(3):370–82. https://doi.org/10.1002/ana.25678.
Szwed K, Słomka A, Pawliszak W, Szwed M, Anisimowicz L, Żekanowska E, et al. Novel markers for predicting type 2 neurologic complications of coronary artery bypass grafting. Ann Thorac Surg. 2020;110(2):599–607. https://doi.org/10.1016/j.athoracsur.2019.10.071.
Ibáñez C, Cifuentes A, Simó C. Recent advances and applications of metabolomics to investigate neurodegenerative diseases. Int Rev Neurobiol. 2015;122:95–132. https://doi.org/10.1016/bs.irn.2015.05.015.
An Y, Varma VR, Varma S, Casanova R, Dammer E, Pletnikova O, et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018;14(3):318–29. https://doi.org/10.1016/j.jalz.2017.09.011.
Zhu B, Shen J, Jiang R, Jin L, Zhan G, Liu J, et al. Abnormalities in gut microbiota and serum metabolites in hemodialysis patients with mild cognitive decline: a single-center observational study. Psychopharmacology. 2020;237(9):2739–52. https://doi.org/10.1007/s00213-020-05569-x.
Johnson LA, Zuloaga KL, Kugelman TL, Mader KS, Morré JT, Zuloaga DG, et al. Amelioration of metabolic syndrome-associated cognitive impairments in mice via a reduction in dietary fat content or infusion of non-diabetic plasma. EBioMedicine. 2016;3:26–42. https://doi.org/10.1016/j.ebiom.2015.12.008.
Dall’Armi C, Devereaux KA, Di Paolo G. The role of lipids in the control of autophagy. Curr Biol. 2013;23(1):R33-45. https://doi.org/10.1016/j.cub.2012.10.041.
Huynh K, Lim WLF, Giles C, Jayawardana KS, Salim A, Mellett NA, et al. Concordant peripheral lipidome signatures in two large clinical studies of Alzheimer’s disease. Nat Commun. 2020;11(1):5698. https://doi.org/10.1038/s41467-020-19473-7.
Xu X, Zhang A, Zhu Y, He W, Di W, Fang Y, et al. MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κB and PI3K-Akt pathways. J Cell Physiol. 2018;234(1):904–14. https://doi.org/10.1002/jcp.26918.
Olsen ASB, Færgeman NJ. Sphingolipids: membrane microdomains in brain development, function and neurological diseases. Open Biol. 2017;7(5). https://doi.org/10.1098/rsob.170069
Cantuti-Castelvetri L, Bongarzone ER. Synaptic failure: the achilles tendon of sphingolipidoses. J Neurosci Res. 2016;94(11):1031–6. https://doi.org/10.1002/jnr.23753.
Savica R, Murray ME, Persson XM, Kantarci K, Parisi JE, Dickson DW, et al. Plasma sphingolipid changes with autopsy-confirmed Lewy Body or Alzheimer’s pathology. Alzheimers Dement (Amst). 2016;3:43–50. https://doi.org/10.1016/j.dadm.2016.02.005.
Mielke MM, Bandaru VV, Haughey NJ, Xia J, Fried LP, Yasar S, et al. Serum ceramides increase the risk of Alzheimer disease: the Women’s Health and Aging Study II. Neurology. 2012;79(7):633–41. https://doi.org/10.1212/WNL.0b013e318264e380.
Acknowledgements
The authors thank Oebiotech (Shanghai, China) for technical assistance in the analysis of the metabolomics data.
Funding
This work was supported by the National Natural Science Foundation of China (81901100), Jiangsu Province Special Program for Young Medical Talent (QNRC2016587) to H.H., and Nanjing Introduction Plan of Leading Technology Entrepreneurship Talents (2014B06011) to Y.C.
Author information
Authors and Affiliations
Contributions
Jingjing Han and He Huang analyzed the data and wrote the initial manuscript. Zheng Lei and Rui Pan made the tables and figures. Xiaodong Chen revised the manuscript. Yu Chen and Ting Lu supervised and provided critical comments on the manuscript. All authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Ethic Approval
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
Consent to Participate
The patients/participants provided written informed consent to participate in this study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Craig M. Stolen oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, J., Huang, H., Lei, Z. et al. Association Between the Early Serum Lipid Metabolism Profile and Delayed Neurocognitive Recovery After Cardiopulmonary Bypass in Cardiac Surgical Patients: a Pilot Study. J. of Cardiovasc. Trans. Res. 16, 662–673 (2023). https://doi.org/10.1007/s12265-022-10332-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10332-y