Abstract
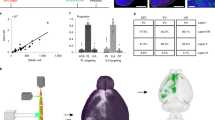
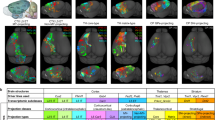
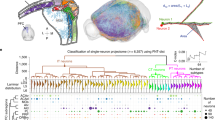
The orbitofrontal cortex (OFC) is involved in diverse brain functions via its extensive projections to multiple target regions. There is a growing understanding of the overall outputs of the OFC at the population level, but reports of the projection patterns of individual OFC neurons across different cortical layers remain rare. Here, by combining neuronal sparse and bright labeling with a whole-brain florescence imaging system (fMOST), we obtained an uninterrupted three-dimensional whole-brain dataset and achieved the full morphological reconstruction of 25 OFC pyramidal neurons. We compared the whole-brain projection targets of these individual OFC neurons in different cortical layers as well as in the same cortical layer. We found cortical layer-dependent projections characterized by divergent patterns for information delivery. Our study not only provides a structural basis for understanding the principles of laminar organizations in the OFC, but also provides clues for future functional and behavioral studies on OFC pyramidal neurons.







Similar content being viewed by others
References
Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. A mesoscale connectome of the mouse brain. Nature 2014, 508: 207–214.
Zingg B, Hintiryan H, Gou L, Song Monica Y, Bay M, Bienkowski Michael S, et al. Neural networks of the mouse neocortex. Cell 2014, 156: 1096–1111.
Han Y, Kebschull JM, Campbell RAA, Cowan D, Imhof F, Zador AM, et al. The logic of single-cell projections from visual cortex. Nature 2018, 556: 51–56.
Lin HM, Kuang JX, Sun P, Li N, Lv X, Zhang YH. Reconstruction of intratelencephalic neurons in the mouse secondary motor cortex reveals the diverse projection patterns of single neurons. Front Neuroanat 2018, 12: 86.
Winnubst J, Bas E, Ferreira TA, Wu Z, Economo MN, Edson P, et al. Reconstruction of 1,000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell 2019, 179: 268–281.e13.
Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett 2008, 432: 40–45.
Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron 2006, 51: 495–507.
Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol 2011, 519: 3766–3801.
Kuramoto E, Iwai H, Yamanaka A, Ohno S, Seki H, Tanaka YR, et al. Dorsal and ventral parts of thalamic nucleus submedius project to different areas of rat orbitofrontal cortex: A single neuron-tracing study using virus vectors. J Comp Neurol 2017, 525: 3821–3839.
Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JFW, et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology 2002, 160: 290–298.
Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci 2004, 24: 7540–7548.
O’Doherty J. Can’t learn without you: predictive value coding in orbitofrontal cortex requires the basolateral amygdala. Neuron 2003, 39: 731–733.
Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. Separate neural pathways process different decision costs. Nat Neurosci 2006, 9: 1161–1168.
Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron 2005, 47: 633–636.
Atmaca M, Yildirim H, Ozdemir H, Tezcan E, Poyraz AK. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007, 31:46–52.
Hou J, Wu W, Lin Y, Wang J, Zhou D, Guo J, et al. Localization of cerebral functional deficits in patients with obsessive–compulsive disorder: A resting-state fMRI study. J Affect Disord 2012, 138: 313–321.
Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci 2007, 1121: 499–527.
Lee SH, Payne ME, Steffens DC, Mcquoid DR, Lai TJ, Provenzale JM, et al. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry 2003, 54: 529–533.
Wang Q, Poh JS, Wen DJ, Broekman BFP, Chong YS, Yap F, et al. Functional and structural networks of lateral and medial orbitofrontal cortex as potential neural pathways for depression in childhood. Depress Anxiety 2019, 36: 365–374.
Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol 2001, 11: 240–249.
Northoff G, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, et al. Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: A combined fMRI/MEG study. Cereb Cortex 2000, 10: 93–107.
Qi X, Du ZJ, Zhu L, Liu X, Xu H, Zhou Z, et al. The glutamatergic postrhinal cortex-ventrolateral orbitofrontal cortex pathway regulates spatial memory retrieval. Neurosci Bull 2019, 35: 447–460.
Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 1995, 363: 615–641.
Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 2004, 28: 771–784.
Haber SN, Kunishio K, Mizobuchi M, Lyndbalta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 1995, 15: 4851–4867.
Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkey. J Comp Neurol 1993, 337: 1–31.
Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry—An update. J Psychosom Res 2002, 53: 647–654.
Bedwell SA, Billett EE, Crofts JJ, MacDonald DM, Tinsley CJ. The topology of connections between rat prefrontal and temporal cortices. Front Syst Neurosci 2015, 9: 80.
Bedwell SA, Billett EE, Crofts JJ, Tinsley CJ. The topology of connections between rat prefrontal, motor and sensory cortices. Front Syst Neurosci 2014, 8: 177.
Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol 2000, 422: 556–578.
Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol 2001, 432: 307–328.
Goncalves L, Nogueira MI, Shammah-Lagnado SJ, Metzger M. Prefrontal afferents to the dorsal raphe nucleus in the rat. Brain Res Bull 2009, 78: 240–247.
Kondo H, Witter MP. Topographic organization of orbitofrontal projections to the parahippocampal region in rats. J Comp Neurol 2014, 522: 772–793.
Yang T, Zheng T, Shang Z, Wang X, Lv X, Yuan J, et al. Rapid imaging of large tissues using high-resolution stage-scanning microscopy. Biomed Opt Express 2015, 6: 1867–1875.
Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009, 459: 698–702.
Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc 2006, 1: 1412–1428.
Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Psychoneuroendocrinology 1997, 28.
Gong H, Xu D, Yuan J, Li X, Guo C, Peng J, et al. High-throughput dual-colour precision imaging for brain-wide connectome with cytoarchitectonic landmarks at the cellular level. Nat Commun 2016, 7: 12142.
Xiong H, Zhou Z, Zhu M, Lv X, Li A, Li S, et al. Chemical reactivation of quenched fluorescent protein molecules enables resin-embedded fluorescence microimaging. Nat Commun 2014, 5: 3992.
Wang X, Zhang Y, Wang X, Dai J, Hua R, Zeng S, et al. Anxiety-related cell-type-specific neural circuits in the anterior-dorsal bed nucleus of the stria terminalis. Sci Bull 2020, 65: 1203–1216.
Gang Y, Zhou H, Jia Y, Liu L, Liu X, Rao G, et al. Embedding and chemical reactivation of green fluorescent protein in the whole mouse brain for optical micro-imaging. Front Neurosci 2017, 11: 121.
Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 2004, 5: 793–807.
Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 2008, 9: 206–221.
Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 2013, 8: e76310.
Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res 2015, 93: 144–157.
Quan T, Zhou H, Li J, Li S, Li A, Li Y, et al. NeuroGPS-Tree: automatic reconstruction of large-scale neuronal populations with dense neurites. Nat Methods 2016, 13: 51–54.
Harris KD, Shepherd GM. The neocortical circuit: themes and variations. Nat Neurosci 2015, 18: 170–181.
Shepherd GMG. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 2013, 14: 278–291.
Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry 1994, 51: 687–697.
Benkelfat C, Nordahl TE, Semple WE, King AC, Murphy DL, Cohen RM. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. Patients treated with clomipramine. Arch Gen Psychiatry 1987, 47: 840–848.
Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput 2002, 14: 793–817.
Markowitsch HJ, Vandekerckhove MMP, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex 2003, 39: 643–665.
Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, et al. beta CaMKII in lateral habenula mediates core symptoms of depression. Science 2013, 341: 1016–1020.
Ohno S, Kuramoto E, Furuta T, Hioki H, Tanaka YR, Fujiyama F, et al. A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: A single neuron tracing study with viral vectors. Cereb Cortex 2012, 22: 2840–2857.
Rodriguez-Moreno J, Rollenhagen A, Arlandis J, Santuy A, Merchan-Perez A, DeFelipe J, et al. Quantitative 3D ultrastructure of thalamocortical synapses from the “lemniscal” ventral posteromedial nucleus in mouse barrel cortex. Cereb Cortex 2018, 28: 3159–3175.
Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 2013, 80: 1368–1383.
van Aerde KI, Feldmeyer D. Morphological and physiological characterization of pyramidal neuron subtypes in rat medial prefrontal cortex. Cereb Cortex 2015, 25: 788–805.
Guo C, Peng J, Zhang Y, Li A, Li Y, Yuan J, et al. Single-axon level morphological analysis of corticofugal projection neurons in mouse barrel field. Sci Rep 2017, 7: 2846.
Zhang Y, Jiang S, Xu Z, Gong H, Li A, Luo Q, et al. Pinpointing morphology and projection of excitatory neurons in mouse visual cortex. Front Neurosci 2019, 13: 912.
Kita T, Kita H. The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci 2012, 32: 5990–5999.
Kampa BM, Letzkus JJ, Stuart GJ. Cortical feed-forward networks for binding different streams of sensory information. Nat Neurosci 2006, 9: 1472–1473.
Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature 2009, 457: 1142–1145.
Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science 2013, 340: 1591–1594.
Feldmeyer D. Excitatory neuronal connectivity in the barrel cortex. Front Neuroanat 2012, 6: 24.
Acknowledgements
We thank the Optical Bioimaging Core Facility of WNLO-HUST and the Analytical and Testing Center of HUST for support in data acquisition. This work was supported by the National Natural Science Foundation of China (61827825, 31770924, 31470056, and 31600692), the Science Fund for Creative Research Group of China (61721092), and the Director Fund of Wuhan National Laboratory for Optoelectronics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Sun, P., Lv, X. et al. Divergent Projection Patterns Revealed by Reconstruction of Individual Neurons in Orbitofrontal Cortex. Neurosci. Bull. 37, 461–477 (2021). https://doi.org/10.1007/s12264-020-00616-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-020-00616-1