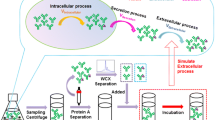
Abstract
Biopharmaceuticals are complex therapeutic protein molecules produced in living cells and have been a major driving force for drug development in the pharmaceutical sector in recent years. Monoclonal antibodies (mAbs) are biological macromolecules used for treating life-threatening and rare illnesses. mAbs with post-translation alterations can be observed during the assessment of charge variants. Controlling the charge variant profile of therapeutic protein is a regulatory requirement to confirm that the macromolecule complies with the quality parameters to ensure patient safety. Unfortunately, manufacturing these biopharmaceuticals is very expensive. However, the emergence of biosimilars has reduced developmental cost across the biopharmaceutical industry. The advent of biosimilars has constrained the development of more efficient downstream bioprocesses that are mainly considered the bottleneck of the manufacturing process. This review focuses on the existing methods for charge variants separation and process optimization and indicates new approaches for future developments. It also provides a comprehensive summary for the biological community about the impact of charge variants.
Similar content being viewed by others
References
Oskouei, S. T. and A. R. Kusmierczyk (2021) Biosimilar uptake: the importance of healthcare provider education. Pharmaceut. Med. 35: 215–224.
Bhunia, B., B. Basak, T. Mandal, P. Bhattacharya, and A. Dey (2013) Effect of pH and temperature on stability and kinetics of novel extracellular serine alkaline protease (70kDa). Int. J. Biol. Macromol. 54: 1–8.
Joshi, S., S. Kumari, and A. S. Rathore (2021) Identification and characterization of carbonylation sites in trastuzumab biosimilars. Int. J. Biol. Macromol. 169: 95–102.
Reslan, M., V. Sifniotis, E. Cruz, Z. Sumer-Bayraktar, S. Cordwell, and V. Kayser (2020) Enhancing the stability of adalimumab by engineering additional glycosylation motifs. Int. J. Biol. Macromol. 158: 189–196.
Beck, A., T. Wurch, C. Bailly, and N. Corvaia (2010) Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 10: 345–352.
Perobelli, R. F., B. Xavier, A. R. da Silveira, G. L. Remuzzi, L. G. J. Motta, and S. L. Dalmora (2018) Quantitation of the monoclonal antibody Denosumab by bioassay and validated LC methods. Int. J. Biol. Macromol. 119: 96–104.
McAtee, C. P. and J. Hornbuckle (2012) Isolation of monoclonal antibody charge variants by displacement chromatography. Curr. Protoc. Protein Sci. 69: 8.10.1–8.10.13.
Kadkhoda, J., M. Akrami-Hasan-Kohal, M. R. Tohidkia, S. Khaledi, S. Davaran, and A. Aghanejad (2021) Advances in antibody nanoconjugates for diagnosis and therapy: A review of recent studies and trends. Int. J. Biol. Macromol. 185: 664–678.
Lu, R.-M., Y.-C. Hwang, I.-J. Liu, C.-C. Lee, H.-Z. Tsai, H.-J. Li, and H.-C. Wu (2020) Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 27: 1.
Berkowitz, S. A., J. R. Engen, J. R. Mazzeo, and G. B. Jones (2012) Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat. Rev. Drug Discov. 11: 527–540.
Vlasak, J., M. C. Bussat, S. Wang, E. Wagner-Rousset, M. Schaefer, C. Klinguer-Hamour, M. Kirchmeier, N. Corvaïa, R. Ionescu, and A. Beck (2009) Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal. Biochem. 392: 145–154.
Khawli, L. A., S. Goswami, R. Hutchinson, Z. W. Kwong, J. Yang, X. Wang, Z. Yao, A. Sreedhara, T. Cano, D. Tesar, I. Nijem, D. E. Allison, P. Y. Wong, Y.-H. Kao, C. Quan, A. Joshi, R. J. Harris, and P. Motchnik (2010) Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. mAbs. 2: 613–624.
Shen, Z., Y. Wang, H. Xu, Q. Zhang, C. Sha, B. Sun, and Q. Li (2021) Analytical comparability assessment on glycosylation of ziv-aflibercept and the biosimilar candidate. Int. J. Biol. Macromol. 180: 494–509.
Simoens, S. and A. G. Vulto (2021) A health economic guide to market access of biosimilars. Expert Opin. Biol. Ther. 21: 9–17.
Agbogbo, F. K., D. M. Ecker, A. Farrand, K. Han, A. Khoury, A. Martin, J. McCool, U. Rasche, T. D. Rau, D. Schmidt, M. Sha, and N. Treuheit (2019) Current perspectives on biosimilars. J. Ind. Microbiol. Biotechnol. 46: 1297–1311.
Niazi, S. K. (2019) Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process. 3rd ed., pp. 95–100. CRC Press.
Vanam, R. P., M. A. Schneider, and M. S. Marlow (2015) Rapid quantitative analysis of monoclonal antibody heavy and light chain charge heterogeneity. mAbs. 7: 1118–1127.
Neill, A., C. Nowak, R. Patel, G. Ponniah, N. Gonzalez, D. Miano, and H. Liu (2015) Characterization of recombinant monoclonal antibody charge variants using OFFGEL fractionation, weak anion exchange chromatography, and mass spectrometry. Anal. Chem. 87: 6204–6211.
Zheng, J. Y. and L. J. Janis (2006) Influence of pH, buffer species, and storage temperature on physicochemical stability of a humanized monoclonal antibody LA298. Int. J. Pharm. 308: 46–51.
Brown, K. A., S. Rajendran, J. Dowd, and D. J. Wilson (2019) Rapid characterization of structural and functional similarity for a candidate bevacizumab (Avastin) biosimilar using a multipronged mass-spectrometry-based approach. Drug Test Anal. 11: 1207–1217.
Majumder, S. K., M. K. Sharma, and T. K. Gupta (2011) Liquid formulation of follicle stimulating hormone. European Patent EP2533800B1.
Majumder, S. K., and T. K. Gupta (2014) Process for the purification of fc fusion. WIPO (PCT) WO2014102814A1.
Du, Y., A. Walsh, R. Ehrick, W. Xu, K. May, and H. Liu (2012) Chromatographic analysis of the acidic and basic species of recombinant monoclonal antibodies. mAbs. 4: 578–585.
Cramer, S. M. and M. A. Holstein (2011) Downstream bioprocessing: recent advances and future promise. Curr. Opin. Chem. Eng. 1: 27–37.
Chon, J. H. and G. Zarbis-Papastoitsis (2011) Advances in the production and downstream processing of antibodies. New Biotechnol. 28: 458–463.
Shukla, A. A. and J. Thömmes (2010) Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol. 28: 253–261.
Rathore, A. S., R. Bhambure, and V. Ghare (2010) Process analytical technology (PAT) for biopharmaceutical products. Anal. Bioanal. Chem. 398: 137–154.
Li, F., Y. Hashimura, R. Pendleton, J. Harms, E. Collins, and B. Lee (2006) A systematic approach for scale-down model development and characterization of commercial cell culture processes. Biotechnol. Prog. 22: 696–703.
Yoo, E. M., K. R. Chintalacharuvu, M. L. Penichet, and S. L. Morrison (2002) Myeloma expression systems. J. Immunol. Methods 261: 1–20.
Costa, A. R., M. E. Rodrigues, M. Henriques, J. Azeredo, and R. Oliveira (2010) Guidelines to cell engineering for monoclonal antibody production. Eur. J. Pharm. Biopharm. 74: 127–138.
Jordan, M., D. Voisard, A. Berthoud, L. Tercier, B. Kleuser, G. Baer, and H. Broly (2013) Cell culture medium improvement by rigorous shuffling of components using media blending. Cytotechnology 65: 31–40.
Li, F., N. Vijayasankaran, A. (Yijuan) Shen, R. Kiss, and A. Amanullah (2010) Cell culture processes for monoclonal antibody production. mAbs. 2: 466–479.
Liu, H. F., J. Ma, C. Winter, and R. Bayer (2010) Recovery and purification process development for monoclonal antibody production. mAbs. 2: 480–499.
Fahrner, R. L., H. L. Knudsen, C. D. Basey, W. Galan, D. Feuerhelm, M. Vanderlaan, and G. S. Blank (2001) Industrial purification of pharmaceutical antibodies: development, operation, and validation of chromatography processes. Biotechnol. Genet. Eng. Rev. 18: 301–327.
Manning, M. C., D. K. Chou, B. M. Murphy, R. W. Payne, and D. S. Katayama (2010) Stability of protein pharmaceuticals: an update. Pharm. Res. 27: 544–575.
Talebi, M., A. Nordborg, A. Gaspar, N. A. Lacher, Q. Wang, X. Z. He, P. R. Haddad, and E. F. Hilder (2013) Charge heterogeneity profiling of monoclonal antibodies using low ionic strength ion-exchange chromatography and well-controlled pH gradients on monolithic columns. J. Chromatogr. A 1317: 148–154.
Wagner-Rousset, E., S. Fekete, L. Morel-Chevillet, O. Colas, N. Corvaïa, S. Cianférani, D. Guillarme, and A. Beck (2017) Development of a fast workflow to screen the charge variants of therapeutic antibodies. J. Chromatogr. A 1498: 147–154.
Liu H., G. Gaza-Bulseco, D. Faldu, C. Chumsae, and J. Sun (2008) Heterogeneity of monoclonal antibodies. J. Pharm. Sci. 97: 2426–2447.
Dick Jr, L. W., D. Qiu, D. Mahon, M. Adamo, and K.-C. Cheng (2008) C-terminal lysine variants in fully human monoclonal antibodies: investigation of test methods and possible causes. Biotechnol. Bioeng. 100: 1132–1143.
Yüce, M., F. Sert, M. Torabfam, A. Parlar, B. Gürel, N. Çakır, D. E. Dağlıkoca, M. A. Khan, and Y. Çapan (2021) Fractionated charge variants of biosimilars: a review of separation methods, structural and functional analysis. Anal. Chim. Acta 1152: 238189.
Perkins, M., R. Theiler, S. Lunte, and M. Jeschke (2000) Determination of the origin of charge heterogeneity in a murine monoclonal antibody. Pharm. Res. 17: 1110–1117.
Yan, B., S. Steen, D. Hambly, J. Valliere-Douglass, T. V. Bos, S. Smallwood, Z. Yates, T. Arroll, Y. Han, H. Gadgil, R. F. Latypov, A. Wallace, A. Lim, G. R. Kleemann, W. Wang, and A. Balland (2009) Succinimide formation at Asn 55 in the complementarity determining region of a recombinant monoclonal antibody IgG1 heavy chain. J. Pharm. Sci. 98: 3509–3521.
Singh, S. K., G. Narula, and A. S. Rathore (2016) Should charge variants of monoclonal antibody therapeutics be considered critical quality attributes? Electrophoresis 37: 2338–2346.
Saxena, A., M. Kumar, B. P. Tripathi, and V. K. Shahi (2010) Organic-inorganic hybrid charged membranes for proteins separation: isoelectric separation of proteins under coupled driving forces. Sep. Purif. Technol. 70: 280–290.
Fekete, S., A. Beck, J. Fekete, and D. Guillarme (2015) Method development for the separation of monoclonal antibody charge variants in cation exchange chromatography Part II: pH gradient approach. J. Pharm. Biomed. Anal. 102: 282–289.
Fekete, S., A. Beck, J.-L. Veuthey, and D. Guillarme (2015) Ion-exchange chromatography for the characterization of biopharmaceuticals. J. Pharm. Biomed. Anal. 113: 43–55.
Urmann, M., H. Graalfs, M. Joehnck, L. R. Jacob, and C. Frech (2010) Cation-exchange chromatography of monoclonal antibodies: characterisation of a novel stationary phase designed for production-scale purification. mAbs. 2: 395–404.
Chung, S., J. Tian, Z. Tan, J. Chen, J. Lee, M. Borys, and Z. J. Li (2018) Industrial bioprocessing perspectives on managing therapeutic protein charge variant profiles. Biotechnol. Bioeng. 115: 1646–1665.
Jing, S.-Y., J.-X. Gou, D. Gao, H.-B. Wang, S.-J. Yao, and D.-Q. Lin (2020) Separation of monoclonal antibody charge variants using cation exchange chromatography: resins and separation conditions optimization. Sep. Purif. Technol. 235: 116136.
Baek, J., A. B. Schwahn, S. Lin, C. A. Pohl, M. De Pra, S. M. Tremintin, and K. Cook (2020) New insights into the chromatography mechanisms of ion-exchange charge variant analysis: dispelling myths and providing guidance for robust method optimization. Anal. Chem. 92: 13411–13419.
Svasti, J. and C. Milstein (1972) The disulphide bridges of a mouse immunoglobulin G1 protein. Biochem. J. 126: 837–850.
Khanal, O., V. Kumar, K. Westerberg, F. Schlegel, and A. M. Lenhoff (2019) Multi-column displacement chromatography for separation of charge variants of monoclonal antibodies. J. Chromatogr. A 1586: 40–51.
Zhang, L., T. Patapoff, D. Farnan, and B. Zhang (2013) Improving pH gradient cation-exchange chromatography of monoclonal antibodies by controlling ionic strength. J. Chromatogr. A 1272: 56–64.
Lee, Y. F., M. Jöhnck, and C. Frech (2018) Evaluation of differences between dual salt-pH gradient elution and mono gradient elution using a thermodynamic model: Simultaneous separation of six monoclonal antibody charge and size variants on preparative-scale ion exchange chromatographic resin. Biotechnol. Prog. 34: 973–986.
Liu, Z., S. R. Wickramasinghe, and X. Qian (2017) Membrane chromatography for protein purifications from ligand design to functionalization. Sep. Sci. Technol. 52: 299–319.
Hintersteiner, B., N. Lingg, E. Janzek, O. Mutschlechner, H. Loibner, and A. Jungbauer (2016) Microheterogeneity of therapeutic monoclonal antibodies is governed by changes in the surface charge of the protein. Biotechnol. J. 11: 1617–1627.
Huang, L., J. Lu, V. J. Wroblewski, J. M. Beals, and R. M. Riggin (2005) In vivo deamidation characterization of monoclonal antibody by LC/MS/MS. Anal. Chem. 77: 1432–1439.
Gaza-Bulseco, G., S. Faldu, K. Hurkmans, C. Chumsae, and H. Liu (2008) Effect of methionine oxidation of a recombinant monoclonal antibody on the binding affinity to protein A and protein G. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 870: 55–62.
Rehder, D. S., D. Chelius, A. McAuley, T. M. Dillon, G. Xiao, J. Crouse-Zeineddini, L. Vardanyan, N. Perico, V. Mukku, D. N. Brems, M. Matsumura, and P. V. Bondarenko (2008) Isomerization of a single aspartyl residue of anti-epidermal growth factor receptor immunoglobulin gamma2 antibody highlights the role avidity plays in antibody activity. Biochemistry 47: 2518–2530.
Alt, N., T. Y. Zhang, P. Motchnik, R. Taticek, V. Quarmby, T. Schlothauer, H. Beck, T. Emrich, and R. J. Harris (2016) Determination of critical quality attributes for monoclonal antibodies using quality by design principles. Biologicals 44: 291–305.
Lee, N., J. J. Lee, H. Yang, S. Baek, S. Kim, S. Kim, T. Lee, D. Song, and G. Park (2019) Evaluation of similar quality attribute characteristics in SB5 and reference product of adalimumab. mAbs. 11: 129–144.
Singh, S. K., D. Kumar, H. Malani, and A. S. Rathore (2021) LC-MS based case-by-case analysis of the impact of acidic and basic charge variants of bevacizumab on stability and biological activity. Sci. Rep. 11: 2487.
Sule, S. V., J. E. Fernandez, V. J. Mecozzi, Y. Kravets, W. C. Yang, P. Feng, S. Liu, L. Zang, A. D. Capili, T. B. Estey, and K. Gupta (2017) Assessing the impact of charge variants on stability and viscosity of a high concentration antibody formulation. J. Pharm. Sci. 106: 3507–3514.
Linke, T., J. Feng, K. Yu, H. J. Kim, Z. Wei, Y. Wang, W. K. Wang, and A. K. Hunter (2012) Process scale separation of an anti-CD22 immunotoxin charge variant. J. Chromatogr. A 1260: 120–125.
Kumar, R., D. Vikramachakravarthi, and P. Pal (2014) Production and purification of glutamic acid: a critical review towards process intensification. Chem. Eng. Process. 81: 59–71.
Frost Ebersold, M. and A. L. Zydney (2004) Separation of protein charge variants by ultrafiltration. Biotechnol. Prog. 20: 543–549.
Madadkar, P., U. Umatheva, G. Hale, Y. Durocher, and R. Ghosh (2017) Ultrafast separation and analysis of monoclonal antibody aggregates using membrane chromatography. Anal. Chem. 89: 4716–4720.
Wang, J., J. Zhou, Y. K. Gowtham, S. W. Harcum, and S. M. Husson (2017) Antibody purification from CHO cell supernatant using new multimodal membranes. Biotechnol. Prog. 33: 658–665.
Orr, V., L. Zhong, M. Moo-Young, and C. P. Chou (2013) Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 31: 450–465.
Hart, D. S., C. Harinarayan, G. Malmquist, A. Axén, M. Sharma, and R. van Reis (2009) Surface extenders and an optimal pore size promote high dynamic binding capacities of antibodies on cation exchange resins. J. Chromatogr. A 1216: 4372–4376.
Ichihara, T., T. Ito, and C. Gillespie (2018) Polishing approach with fully connected flow-through purification for therapeutic monoclonal antibody. Eng. Life Sci. 19: 31–36.
Somasundaram, B., K. Pleitt, E. Shave, K. Baker, and L. H. L. Lua (2018) Progression of continuous downstream processing of monoclonal antibodies: current trends and challenges. Biotechnol. Bioeng. 115: 2893–2907.
FDA (2017) Modernizing the way drugs are made: a transition to continuous manufacturing. https://www.fda.gov/drugs/news-events-human-drugs/modernizing-way-drugs-are-made-transition-continuous-manufacturing.
Zhang, J., L. Conley, J. Pieracci, and S. Ghose (2016) Pool-less processing to streamline downstream purification of monoclonal antibodies. Eng. Life Sci. 17: 117–124.
Klutz, S., L. Holtmann, M. Lobedann, and G. Schembecker (2016) Cost evaluation of antibody production processes in different operation modes. Chem. Eng. Sci. 141: 63–74.
Kateja, N., D. Kumar, A. Godara, V. Kumar, and A. S. Rathore (2017) Integrated chromatographic platform for simultaneous separation of charge variants and aggregates from monoclonal antibody therapeutic products. Biotechnol. J.https://doi.org/10.1002/biot.201700133.
Acknowledgements
The authors are thankful to Kashiv BioSciences Pvt. Ltd. and Institute of Science, Nirma University, Ahmedabad, Gujarat for providing us with the necessary facilities and guidance for the completion of the review article.
Author information
Authors and Affiliations
Contributions
Tarun Gupta, Anuj Kumar and Sriram Seshadri performed the initial literature search, all authors contributed to the writing and final editing of the paper. Sriram Seshadri designed the paper and supervised the writing.
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, T., Kumar, A. & Seshadri, S. Bioprocess Challenges in Purification of Therapeutic Protein Charge Variants. Biotechnol Bioproc E 28, 493–506 (2023). https://doi.org/10.1007/s12257-023-0078-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-023-0078-4