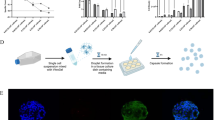
Abstract
Microencapsulation of human mesenchymal stromal cells (MSCs) via electrospraying has been well documented in tissue engineering and regenerative medicine. Herein, we report the use of microencapsulation, via electro-spraying, for MSC expansion using a commercially available hydrogel that is durable, optimized to MSC culture, and enzymatically degradable for cell recovery. Critical parameters of the electrospraying encapsulation process such as seeding density, correlation of microcapsule output with hydrogel volume, and applied voltage were characterized to consistently fabricate cell-laden microcapsules of uniform size. Upon encapsulation, we then verified ∼10× expansion of encapsulated MSCs within a vertical-wheel bioreactor and the preservation of critical quality attributes such as immunophenotype and multipotency after expansion and cell recovery. Finally, we highlight the genetic manipulation of encapsulated MSCs as an example of incorporating bioactive agents in the capsule material to create new compositions of MSCs with altered phenotypes.
Similar content being viewed by others
References
Burr, A. and B. Parekkadan (2019) Kinetics of MSC-based enzyme therapy for immunoregulation. J. Transl. Med. 17: 263.
Li, M., D. Khong, L.-Y. Chin, A. Singleton, and B. Parekkadan (2018) Therapeutic delivery specifications identified through compartmental analysis of a mesenchymal stromal cell-immune reaction. Sci. Rep. 8: 6816.
Zhang, Y., M. Ravikumar, L. Ling, V. Nurcombe, and S. M. Cool (2021) Age-related changes in the inflammatory status of human mesenchymal stem cells: implications for cell therapy. Stem Cell Reports 16: 694–707.
Angelopoulou, M., E. Novelli, J. E. Grove, H. M. Rinder, C. Civin, L. Cheng, and D. S. Krause (2003) Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp. Hematol. 31: 413–420.
Ball, L. M., M. E. Bernardo, H. Roelofs, A. Lankester, A. Cometa, R. M. Egeler, F. Locatelli, and W. E. Fibbe (2007) Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 110: 2764–2767.
Wang, J. F., Y.-F. Wu, J. Harrintong, and I. K. McNiece (2004) Ex vivo expansions and transplantations of mouse bone marrow-derived hematopoietic stem/progenitor cells. J. Zhejiang Univ. Sci. 5: 157–163.
Lin, B. L., J.-F. Chen, W.-H. Qiu, K.-W. Wang, D.-Y. Xie, X.-Y. Chen, Q.-L. Liu, L. Peng, J.-G. Li, Y.-Y. Mei, W.-Z. Weng, Y.-W. Peng, H.-J. Cao, J.-Q. Xie, S.-B. Xie, A. P. Xiang, and Z.-L. Gao (2017) Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology 66: 209–219.
Petrou, P., I. Kassis, N. Levin, F. Paul, Y. Backner, T. Benoliel, F. C. Oertel, M. Scheel, M. Hallimi, N. Yaghmour, T. B. Hur, A. Ginzberg, Y. Levy, O. Abramsky, and D. Karussis (2020) Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 143: 3574–3588.
Kurtzberg, J., S. Prockop, P. Teira, H. Bittencourt, V. Lewis, K. W. Chan, B. Horn, L. Yu, J.-A. Talano, E. Nemecek, C. R. Mills, and S. Chaudhury (2014) Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol. Blood Marrow Transplant. 20: 229–235.
Zhao, K., R. Lou, F. Huang, Y. Peng, Z. Jiang, K. Huang, X. Wu, Y. Zhang, Z. Fan, H. Zhou, C. Liu, Y. Xiao, J. Sun, Y. Li, P. Xiang, and Q. Liu (2015) Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 21: 97–104.
Moon, K.-C., H.-S. Suh, K.-B. Kim, S.-K. Han, K.-W. Young, J.-W. Lee, and M.-H. Kim (2019) Potential of allogeneic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers. Diabetes 68: 837–846.
Braid, L. R., W.-G. Hu, J. E. Davies, and L. P. Nagata (2016) Engineered mesenchymal cells improve passive immune protection against lethal venezuelan equine encephalitis virus exposure. Stem Cells Transl. Med. 5: 1026–1035.
Allen, A., N. Vaninov, M. Li, S. Nguyen, M. Singh, P. Igo, A. W. Tilles, B. O’Rourke, B. L. K. Miller, B. Parekkadan, and R. N. Barcia (2020) Mesenchymal stromal cell bioreactor for ex vivo reprogramming of human immune cells. Sci. Rep. 10: 10142. (Erratum published 2020, Sci. Rep. 10: 15451)
Kink, J. A., M. H. Forsberg, S. Reshetylo, S. Besharat, C. J. Childs, J. D. Pederson, A. Gendron-Fitzpatrick, M. Graham, P. D. Bates, E. G. Schmuck, A. Raval, P. Hematti, and C. M. Capitini (2019) Macrophages educated with exosomes from primed mesenchymal stem cells treat acute radiation syndrome by promoting hematopoietic recovery. Biol. Blood Marrow Transplant. 25: 2124–2133.
Shao, M., Q. Xu, Z. Wu, Y. Chen, Y. Shu, X. Cao, M. Chen, B. Zhang, Y. Zhou, R. Yao, Y. Shi, and H. Bu (2020) Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res. Ther. 11: 37.
Hare, J. M., J. H. Traverse, T. D. Henry, N. Dib, R. K. Strumpf, S. P. Schulman, G. Gerstenblith, A. N. DeMaria, A. E. Denktas, R. S. Gammon, J. B. HermillerJr, M. A. Reisman, G. L. Schaer, and W. Sherman (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 54: 2277–2286.
Olsen, T. R., K. S. Ng, L. T. Lock, T. Ahsan, and J. A. Rowley (2018) Peak MSC-Are we there yet? Front. Med. (Lausanne). 5: 178.
Rowley, J., E. Abraham, A. Campbell, H. Brandwein, and S. Oh (2012) Meeting lot-size challenges of manufacturing adherent cells for therapy. BioProcess Int. 10: 16–22.
Schnitzler, A. C., A. Verma, D. E. Kehoe, D. Jing, J. R. Murrell, K. A. Der, M. Aysola, P. J. Rapiejko, S. Punreddy, and M. S. Rook (2016) Bioprocessing of human mesenchymal stem/stromal cells for therapeutic use: Current technologies and challenges. Biochem. Eng. J. 108: 3–13.
Teryek, M., A. Doshi, L. S. Sherman, P. Rameshwar, S. Jung, and B. Parekkadan (2022) Clinical manufacturing of human mesenchymal stromal cells using a potency-driven paradigm. Curr. Stem Cell Rep. 8: 61–71.
Rafiq, Q. A., K. Coopman, A. W. Nienow, and C. J. Hewitt (2016) Systematic microcarrier screening and agitated culture conditions improves human mesenchymal stem cell yield in bioreactors. Biotechnol. J. 11: 473–486.
Croughan, M. S., D. Giroux, D. Fang, and B. Lee (2016) Chapter 5 - novel single-use bioreactors for scale-up of anchorage-dependent cell manufacturing for cell therapies. pp. 105–139. In: J. M. S. Cabral, C. Lobato de Silva, L. G. Chase, and M. Margarida Diogo (eds.). Stem Cell Manufacturing. Elsevier.
Silva Couto, P., M. C. Rotondi, A. Bersenev, C. J. Hewitt, A. W. Nienow, F. Verter, and Q. A. Rafiq (2020) Expansion of human mesenchymal stem/stromal cells (hMSCs) in bioreactors using microcarriers: lessons learnt and what the future holds. Biotechnol. Adv. 45: 107636.
Tønnesen H. H. and J. Karlsen (2002) Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 28: 621–630.
Davis, M. S., I. Marrero-Berrios, I. Perez, C. P. Rabolli, P. Radhakrishnan, D. Manchikalapati, J. Schianodicola, H. Kamath, R. S. Schloss, and J. Yarmush (2019) Alginate encapsulation for bupivacaine delivery and mesenchymal stromal cell immunomodulatory cotherapy. J. Inflamm. Res. 12: 87–97.
Kumar, S., M. Kabat, S. Basak, J. Babiarz, F. Berthiaume, and M. Grumet (2022) Anti-inflammatory effects of encapsulated human mesenchymal stromal/stem cells and a method to scale-up cell encapsulation. Biomolecules 12: 1803.
Stucky, E. C., J. Erndt-Marino, R. S. Schloss, M. L. Yarmush, and D. I. Shreiber (2017) Prostaglandin E2 produced by alginateencapsulated mesenchymal stromal cells modulates the astrocyte inflammatory response. Nano Life 7: 1750005.
Franco, C. L., J. Price, and J. L. West (2011) Development and optimization of a dual-photoinitiator, emulsion-based technique for rapid generation of cell-laden hydrogel microspheres. Acta Biomater. 7: 3267–3276.
Wilson J. L. and T. C. McDevitt (2013) Stem cell microencapsulation for phenotypic control, bioprocessing, and transplantation. Biotechnol. Bioeng. 110: 667–682.
Huang, L., A. M. E. Abdalla, L. Xiao, and G. Yang (2020) Biopolymer-based microcarriers for three-dimensional cell culture and engineered tissue formation. Int. J. Mol. Sci. 21: 1895.
Wang, Z., D. Wu, J. Zou, Q. Zhou, W. Liu, W. Zhang, G. Zhou, X. Wang, G. Pei, Y. Cao, and Z.-Y. Zhang (2017) Development of demineralized bone matrix-based implantable and biomimetic microcarrier for stem cell expansion and single-step tissue-engineered bone graft construction. J. Mater. Chem. B 5: 62–73.
Divieto, C. and M. P. Sassi (2015) A first approach to evaluate the cell dose in highly porous scaffolds by using a nondestructive metabolic method. Future Sci. OA 1: FSO58.
Bhatt, R., D. Ravi, A. M. Evens, and B. Parekkadan (2022) Scaffold-mediated switching of lymphoma metabolism in culture. Cancer Metab. 10: 15.
Gabusi, E., E. Lenzi, C. Manferdini, P. Dolzani, M. Columbaro, Y. Saleh, and G. Lisignoli (2022) Autophagy is a crucial path in chondrogenesis of adipose-derived mesenchymal stromal cells laden in hydrogel. Gels 8: 766.
Collon, K., M. C. Gallo, J. A. Bell, S. W. Chang, J. C. S. Rodman, O. Sugiyama, D. B. Kohn, and J. R. Lieberman (2022) Improving lentiviral transduction of human adipose-derived mesenchymal stem cells. Hum. Gene Ther. 33: 1260–1268.
Neshati, V., S. Mollazadeh, B. S. Fazly Bazzaz, A. A. de Vries, M. Mojarrad, H. Naderi-Meshkin, Z. Neshati, and M. A. Kerachian (2018) Cardiomyogenic differentiation of human adipose-derived mesenchymal stem cells transduced with Tbx20-encoding lentiviral vectors. J. Cell. Biochem. 119: 6146–6153.
Mangi, A. A., N. Noiseux, D. Kong, H. He, M. Rezvani, J. S. Ingwall, and V. J. Dzau (2003) Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 9: 1195–1201.
Kumar, S. and S. Ponnazhagan (2007) Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 21: 3917–3927.
Amari, A., M. Ebtekar, S. M. Moazzeni, M. Soleimani, L. Mohammadi Amirabad, M. T. Tahoori, and M. Massumi (2015) In vitro generation of IL-35-expressing human Wharton’s jelly-derived mesenchymal stem cells using lentiviral vector. Iran. J. Allergy Asthma Immunol. 14: 416–426.
Zhu, Y., M. Cheng, Z. Yang, C.-Y. Zeng, J. Chen, Y. Xie, S.-W. Luo, K.-H. Zhang, S.-F. Zhou, and N.-H. Lu (2014) Mesenchymal stem cell-based NK4 gene therapy in nude mice bearing gastric cancer xenografts. Drug Des. Devel. Ther. 8: 2449–2462.
Goh, T. K., Z.-Y. Zhang, A. K. Chen, S. Reuveny, M. Choolani, J. K. Chan, and S. K. Oh (2013) Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores. Open Access 2: 84–97.
Heathman, T. R., A. Stolzing, C. Fabian, Q. A. Rafiq, K. Coopman, A. W. Nienow, B. Kara, and C. J. Hewitt (2016) Scalability and process transfer of mesenchymal stromal cell production from monolayer to microcarrier culture using human platelet lysate. Cytotherapy 18: 523–535.
Hervy, M., J. L. Weber, M. Pecheul, P. Dolley-Sonneville, D. Henry, Y. Zhou, and Z. Melkoumian (2014) Long term expansion of bone marrow-derived hMSCs on novel synthetic microcarriers in xeno-free, defined conditions. PLoS One 9: e92120.
Jain, E., K. M. Scott, S. P. Zustiak, and S. A. Sell (2015) Fabrication of polyethylene glycol-based hydrogel microspheres through electrospraying. Macromol. Mater. Eng. 300: 823–835.
Mumaw, J., E. T. Jordan, C. Sonnet, R. M. Olabisi, E. A. Olmsted-Davis, A. R. Davis, J. F. Peroni, J. L. West, F. West, Y. Lu, and S. L. Stice (2012) Rapid heterotrophic ossification with cryopreserved poly(ethylene glycol-) microencapsulated BMP2-expressing MSCs. Int. J. Biomater. 2012: 861794.
Perera, D., M. Medini, D. Seethamraju, R. Falkowski, K. White, and R. M. Olabisi (2018) The effect of polymer molecular weight and cell seeding density on viability of cells entrapped within PEGDA hydrogel microspheres. J. Microeneapsul. 35: 475–481.
Heathman, T. R. J., Q. A. Rafiq, A. K. C. Chan, K. Coopman, A. W. Nienow, B. Kara, and C. J. Hewitt (2016) Characterization of human mesenchymal stem cells from multiple donors and the implications for large scale bioprocess development. Biochem. Eng. J. 108: 14–23.
Siegel, G., T. Kluba, U. Hermanutz-Klein, K. Bieback, H. Northoff, and R. Schäfer (2013) Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 11: 146.
Gryshkov, O., D. Pogozhykh, H. Zernetsch, N. Hofmann, T. Mueller, and B. Glasmacher (2014) Process engineering of high voltage alginate encapsulation of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 36: 77–83.
Qayyum, A. S., E. Jain, G. Kolar, Y. Kim, S. A. Sell, and S. P. Zustiak (2017) Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication 9: 025019.
Lembong, J., R. Kirian, J. D. Takacs, T. R. Olsen, L. T. Lock, J. A. Rowley, and T. Ahsan (2020) Bioreactor parameters for microcarrier-based human MSC expansion under xeno-free conditions in a vertical-wheel system. Bioengineering (Basel) 7: 73.
Rafiq, Q. A., S. Ruck, M. P. Hanga, T. R. J. Heathman, K. Coopman, A. W. Nienow, D. J. Williams, and C. J. Hewitt (2018) Qualitative and quantitative demonstration of bead-to-bead transfer with bone marrow-derived human mesenchymal stem cells on microcarriers: utilising the phenomenon to improve culture performance. Biochem. Eng. J. 135: 11–21.
Lam, A. T., J. Li, A. K.-L. Chen, S. Reuveny, S. K.-W. Oh, and W. R. Birch (2014) Cationic surface charge combined with either vitronectin or laminin dictates the evolution of human embryonic stem cells/microcarrier aggregates and cell growth in agitated cultures. Stem Cells Dev. 23: 1688–1703.
Al-Rubeai, M., R. P. Singh, M. H. Goldman, and A. N. Emery (1995) Death mechanisms of animal cells in conditions of intensive agitation. Biotechnol. Bioeng. 45: 463–472.
Selden, C., J. Bundy, E. Erro, E. Puschmann, M. Miller, D. Kahn, H. Hodgson, B. Fuller, J. Gonzalez-Molina, A. Le Lay, S. Gibbons, S. Chalmers, S. Modi, A. Thomas, P. Kilbride, A. Isaacs, R. Ginsburg, H. Ilsley, D. Thomson, G. Chinnery, N. Mankahla, L. Loo, and C. W. Spearman (2017) A clinical-scale BioArtificial Liver, developed for GMP, improved clinical parameters of liver function in porcine liver failure. Sci. Rep. 7: 14518.
Swioklo, S., P. Ding, A. W. Pacek, and C. J. Connon (2017) Process parameters for the high-scale production of alginate-encapsulated stem cells for storage and distribution throughout the cell therapy supply chain. Process Biochem. 59: 289–296.
Schop, D., F. W. Janssen, L. D. van Rijn, H. Fernandes, R. M. Bloem, J. D. de Bruijn, and R. van Dijkhuizen-Radersma (2009) Growth, metabolism, and growth inhibitors of mesenchymal stem cells. Tissue Eng. Part A 15: 1877–1886.
Pattasseril, J., H. Varadaraju, L. Lock, and J. A. Rowley (2013) Downstream technology landscape for large-scale therapeutic cell processing. BioProcess Int. 11: 38–47.
Pigeau, G. M., E. Csaszar, and A. Dulgar-Tulloch (2018) Commercial scale manufacturing of allogeneic cell therapy. Front. Med. (Lausanne). 5: 233.
Welter, J. F., L. A. Solchaga, and K. J. Penick (2007) Simplification of aggregate culture of human mesenchymal stem cells as a chondrogenic screening assay. Biotechniques 42: 732, 734–737.
Panchision, D. M., H.-L. Chen, F. Pistollato, D. Papini, H.-T. Ni, and T. S. Hawley (2007) Optimized flow cytometric analysis of central nervous system tissue reveals novel functional relationships among cells expressing CD133, CD15, and CD24. Stem Cells 25: 1560–1570.
Santos, J. L., D. Pandita, J. Rodrigues, A. P. Pêgo, P. L. Granja, and H. Tomás (2011) Non-viral gene delivery to mesenchymal stem cells: methods, strategies and application in bone tissue engineering and regeneration. Curr. Gene Ther. 11: 46–57.
Marofi, F., G. Vahedi, A. Biglari, A. Esmaeilzadeh, and S. S. Athari (2017) Mesenchymal stromal/stem cells: a new era in the cell-based targeted gene therapy of cancer. Front. Immunol. 8: 1770.
Lin, P., Y. Lin, D. P. Lennon, D. Correa, M. Schluchter, and A. I. Caplan (2012) Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Transl. Med. 1: 886–897.
Andrews, S., A. Cheng, H. Stevens, M. T. Logun, R. Webb, E. Jordan, B. Xia, L. Karumbaiah, R. E. Guldberg, and S. Stice (2019) Chondroitin sulfate glycosaminoglycan scaffolds for cell and recombinant protein-based bone regeneration. Stem Cells Transl. Med. 8: 575–585.
Dimitrov, D. S. (2004) Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2: 109–122.
Amadeo, F., V. Hanson, P. Murray, and A. Taylor (2022) DEAE-dextran enhances the lentiviral transduction of primary human mesenchymal stromal cells from all major tissue sources without affecting their proliferation and phenotype. Mol. Biotechnol. 65: 544–555.
Acknowledgements
This research was conducted with support by under Contract number T0067 from the Advanced Regenerative Medicine Institute, BioFAB USA and Grant Nos. R01EB012521 (BP) and R01EB02872 (BP) awarded by the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conceptualization: BP, MT. Execution of Experiments: MT, PJ, RB. Formal Analysis: MT, PJ, RB. Manuscript Preparation: MT, PJ, RB. Funding Acquisition: BP.
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
No ethical approval required.
Single donor bone marrow was donated for purchase from Lonza (Walkersville, MD, USA). Lonza obtained permission for its use in research applications by written informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Teryek, M., Jadhav, P., Bento, R. et al. High-Throughput Production of Microcapsules for Human Bone Marrow Derived Mesenchymal Stem Cell Biomanufacturing in a Vertical-Wheel Bioreactor. Biotechnol Bioproc E 28, 528–544 (2023). https://doi.org/10.1007/s12257-023-0020-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-023-0020-9