Abstract
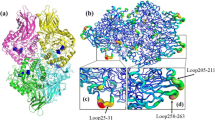
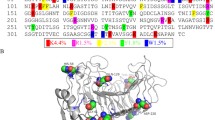
The α-glycosidase from Xanthomonas campestris (XgtA) can specifically catalyze the transglycosylation reactions, thus can be applied for the enzymatic synthesis of α-glycosides. However, the low thermal stability of XgtA has been a bottleneck for its industrial application. In this research, a combined semi-rational design strategy was used based on analysis of the α-helices and β-turns of XgtA, FireProt prediction of thermostable mutants and molecular dynamic simulations for improving the thermostability. The key positions with a significant impact on the thermal stability of XgtA were identified and the effect of proline substitution was tested. Five single-point mutants V167P, A177P, A210P, A220P, T345P, and a combined mutant Mut5 were obtained with improved thermal stability. Mut5 displayed a 3.06-fold increase in time of half-life at 45°C without impairing its initial hydrolytic activity. Molecular dynamics simulations revealed that changes in the flexibility of amino acid residues, newly-forming hydrogen bonding networks and hydrophobic interactions were responsible for the improved thermostability. These results suggest that proline substitution of key flexible positions is an effective strategy for improving enzyme thermostability.
Similar content being viewed by others
References
Kimura, A. (2000) Molecular anatomy of α-glucosidase. Trends Glycosci. Glycotechnol. 12: 373–380.
Desmet, T. W. Soetaert, P. Bojarová, V. Křen, L. Dijkhuizen, V. Eastwick-Field, and A. Schiller (2012) Enzymatic glycosylation of small molecules: challenging substrates require tailored catalysts. Chemistry. 18: 10786–10801.
Lévêque, E., Š. Janeček, B. Haye, and A. Belarbi (2000) Thermophilic archaeal amylolytic enzymes. Enzyme Microb. Technol. 26: 3–14.
Hung, V. S., Y. Hatada, S. Goda, J. Lu, Y. Hidaka, Z. Li, M. Akita, Y. Ohta, K. Watanabe, H. Matsui, S. Ito, and K. Horikoshi (2005) alpha-Glucosidase from a strain of deep-sea Geobacillus: a potential enzyme for the biosynthesis of complex carbohydrates. Appl. Microbiol. Biotechnol. 68: 757–765.
Kato, N., S. Suyama, M. Shirokane, M. Kato, T. Kobayashi, and N. Tsukagoshi (2002) Novel alpha-glucosidase from Aspergillus nidulans with strong transglycosylation activity. Appl. Environ. Microbiol. 68: 1250–1256.
Malá, S., H. Dvoráková, R. Hrabal, and B. Králová (1999) Towards regioselective synthesis of oligosaccharides by use of alpha-glucosidases with different substrate specificity. Carbohydr. Res. 322: 209–218.
Bissaro, B., P. Monsan, R. Fauré, and M. J. O’Donohue (2015) Glycosynthesis in a waterworld: new insight into the molecular basis of transglycosylation in retaining glycoside hydrolases. Biochem. J. 467: 17–35.
Chen, L., Y. Zhou, C. Lu, Z. Ma, H. Chen, L. Zhu, Y. Lu, and X. Chen (2021) Efficient production of l-menthyl α-glucopyranoside from l-menthol via whole-cell biotransformation using recombinant Escherichia coli. Biotechnol. Lett. 43: 1757–1764.
Kurosu, J., T. Sato, K. Yoshida, T. Tsugane, S. Shimura, K. Kirimura, K. Kino, and S. Usami (2002) Enzymatic synthesis of alpha-arbutin by alpha-anomer-selective glucosylation of hydroquinone using lyophilized cells of Xanthomonas campestris WU-9701. J. Biosci. Bioeng. 93: 328–330.
Sato, T., H. Takeuchi, K. Takahashi, J. Kurosu, K. Yoshida, T. Tsugane, S. Shimura, K. King, and K. Kirimura (2003) Selective alpha-glucosylation of eugenol by alpha-glucosyl transfer enzyme of Xanthomonas campestris WU-9701. J. Biosci. Bioeng. 96: 199–202.
Sato, T., H. Nakagawa, J. Kurosu, K. Yoshida, T. Tsugane, S. Shimura, K. Kirimura, K. Kino, and S. Usami (2000) Alpha-anomer-selective glucosylation of (+)-catechin by the crude enzyme, showing glucosyl transfer activity, of Xanthomonas campestris WU-9701. J. Biosci. Bioeng. 90: 625–630.
Watanabe, R., Y. Arimura, Y. Ishii, and K. Kirimura (2020) Crystal structure of α-glucosyl transfer enzyme XgtA from Xanthomonas campestris WU-9701. Biochem. Biophys. Res. Commun. 526: 580–585.
Sato, T., N. Hasegawa, J. Saito, S. Umezawa, Y. Honda, K. Kino, and K. Kirimura (2012) Purification, characterization, and gene identification of an α-glucosyl transfer enzyme, a novel type α-glucosidase from Xanthomonas campestris WU-9701. J. Mol. Catal. B Enzym. 80: 20–27.
Bommarius, A. S. and M. F. Paye (2013) Stabilizing biocatalysts. Chem. Soc. Rev. 42: 6534–6565.
Xu, Z., Y. K. Cen, S. P. Zou, Y. P. Xue, and Y. G. Zheng (2020) Recent advances in the improvement of enzyme thermostability by structure modification. Crit. Rev. Biotechnol. 40: 83–98.
Chen, H., S. Yang, A. Xu, R. Jiang, Z. Tang, J. Wu, L. Zhu, S. Liu, X. Chen, and Y. Lu (2019) Insight into the glycosylation and hydrolysis kinetics of alpha-glucosidase in the synthesis of glycosides. Appl. Microbiol. Biotechnol. 103: 9423–9432.
Talakad, J. C., P. R. Wilderman, D. R. Davydov, S. Kumar, and J. R. Halpert (2010) Rational engineering of cytochromes P450 2B6 and 2B11 for enhanced stability: insights into structural importance of residue 334. Arch. Biochem. Biophys. 494: 151–158.
Reetz, M. T., J. D. Carballeira, and A. Vogel (2006) Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angew. Chem. Int. Ed. Engl. 45: 7745–7751.
Chen, Q., Y. Xiao, W. Zhang, and W. Mu (2020) Current methods and applications in computational protein design for food industry. Crit. Rev. Food Sci. Nutr. 60: 3259–3270.
Schymkowitz, J., J. Borg, F. Stricher, R. Nys, F. Rousseau, and L. Serrano (2005) The FoldX web server: an online force field. Nucleic Acids Res. 33: W382–W388.
Reetz, M. T. and J. D. Carballeira (2007) Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat. Protoc. 2: 891–903.
Zeiske, T., K. A. Stafford, and A. G. Palmer3rd (2016) Thermostability of enzymes from molecular dynamics simulations. J. Chem. Theory Comput. 12: 2489–2492.
Sun, Z., Q. Liu, G. Qu, Y. Feng, and M. T. Reetz (2019) Utility of B-factors in protein science: interpreting rigidity, flexibility, and internal motion and engineering thermostability. Chem. Rev. 119: 1626–1665.
Ge, L., D. Li, T. Wu, L. Zhao, G. Ding, Z. Wang, and W. Xiao (2018) B-factor-saturation mutagenesis as a strategy to increase the thermostability of α-L-rhamnosidase from Aspergillus terreus. J. Biotechnol. 275: 17–23.
Parthasarathy, S. and M. R. N. Murthy (2000) Protein thermal stability: insights from atomic displacement parameters (B values). Protein Eng. 13: 9–13.
Krüger, D. M., P. C. Rathi, C. Pfleger, and H. Gohlke (2013) CNA web server: rigidity theory-based thermal unfolding simulations of proteins for linking structure, (thermo-)stability, and function. Nucleic Acids Res. 41: W340–W348.
Pfleger, C., P. C. Rathi, D. L. Klein, S. Radestock, and H. Gohlke (2013) Constraint Network Analysis (CNA): a Python software package for efficiently linking biomacromolecular structure, flexibility, (thermo-)stability, and function. J. Chem. Inf. Model. 53: 1007–1015.
Matthews, B. W., H. Nicholson, and W. J. Becktel (1987) Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc. Natl. Acad. Sci. U. S. A. 84: 6663–6667.
Hardy, F., G. Vriend, O. R. Veltman, B. van der Vinne, G. Venema, and V. G. H. Eijsink (1993) Stabilization of Bacillus stearothermophilus neutral protease by introduction of prolines. FEBS Lett. 317: 89–92.
Zhu, G. P., C. Xu, M. K. Teng, L. M. Tao, X. Y. Zhu, C. J. Wu, J. Hang, L. W. Niu, and Y. Z. Wang (1999) Increasing the thermostability of D-xylose isomerase by introduction of a proline into the turn of a random coil. Protein Eng. 12: 635–638.
Watanabe, K., T. Masuda, H. Ohashi, H. Mihara, and Y. Suzuki (1994) Multiple proline substitutions cumulatively thermostabilize Bacillus cereus ATCC7064 oligo-1,6-glucosidase. Irrefragable proof supporting the proline rule. Eur. J. Biochem. 226: 277–283.
Fuchs, P. F. J. and A. J. P. Alix (2005) High accuracy prediction of beta-turns and their types using propensities and multiple alignments. Proteins. 59: 828–839.
Anbarasan, S., J. Jänis, M. Paloheimo, M. Laitaoja, M. Vuolanto, J. Karimäki, P. Vainiotalo, M. Leisola, and O. Turunen (2010) Effect of glycosylation and additional domains on the thermostability of a family 10 xylanase produced by Thermopolyspora flexuosa. Appl. Environ. Microbiol. 76: 356–360.
Goihberg, E., O. Dym, S. Tel-Or, I. Levin, M. Peretz, and Y. Burstein (2007) A single proline substitution is critical for the thermostabilization of Clostridium beijerinckii alcohol dehydrogenase. Proteins. 66: 196–204.
Dotsenko, A. S., S. Pramanik, A. V. Gusakov, A. M. Rozhkova, I. N. Zorov, A. P. Sinitsyn, M. D. Davari, and U. Schwaneberg (2019) Critical effect of proline on thermostability of endoglucanase II from Penicillium verruculosum. Biochem. Eng. J. 152: 107395.
Yu, H., Y. Zhao, C. Guo, Y. Gan, and H. Huang (2015) The role of proline substitutions within flexible regions on thermostability of luciferase. Biochim. Biophys. Acta. 1854: 65–72.
Spassov, V. Z. and L. Yan (2016) A pH-dependent computational approach to the effect of mutations on protein stability. J. Comput. Chem. 37: 2573–2587.
Musil, M., J. Stourac, J. Bendl, J. Brezovsky, Z. Prokop, J. Zendulka, T. Martinek, D. Bednar, and J. Damborsky (2017) FireProt: web server for automated design of thermostable proteins. Nucleic Acids Res. 45: W393–W399.
Pace, C. N., H. Fu, K. L. Fryar, J. Landua, S. R. Trevino, B. A. Shirley, M. M. Hendricks, S. Iimura, K. Gajiwala, J. M. Scholtz, and G. R. Grimsley (2011) Contribution of hydrophobic interactions to protein stability. J. Mol. Biol. 408: 514–528.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (82104322) and the Natural Science Foundation of Zhejiang (LQ22H280012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests. Neither ethical approval nor informed consent was required for this study.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chen, L., Jiang, K., Zhou, Y. et al. Improving the Thermostability of α-Glucosidase from Xanthomonas campestris through Proline Substitutions Guided by Semi-rational Design. Biotechnol Bioproc E 27, 631–639 (2022). https://doi.org/10.1007/s12257-022-0129-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-022-0129-2