Summary
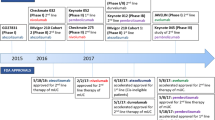
Three trials presented at the meeting investigated the incorporation of immunotherapy into a multimodal bladder-sparing treatment approach. The results of these phase 2 trials are so far promising with most patients achieving complete remission (CR) and estimated bladder-intact disease-free survival (BIDFS) in these patients ranges between 73 and 88%. Additionally, the up to 5-year follow-up data from the KEYNOTE-052 study were discussed. The results confirm the efficacy of pembrolizumab as first-line monotherapy in cisplatin-ineligible patients with a programmed death ligand 1 (PD-L1) combined positive score (CPS) ≥ 10.
Similar content being viewed by others
References
O’Donnell PH, Balar AV, Vuky J, Castellano D, Bellmunt J, Powles T, et al. First-line pembrolizumab (pembro) in cisplatin-ineligible patients with advanced urothelial cancer (UC): response and survival results up to five years from the KEYNOTE-052 phase 2 study. J Clin Oncol. 2021;39(15_suppl):4508.
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine. 2020;383(13):1218–30.
Balar AV, Milowsky MI, O’Donnell PH, Alva AS, Kollmeier M, Rose TL, et al. Pembrolizumab (pembro) in combination with gemcitabine (gem) and concurrent hypofractionated radiation therapy (RT) as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder (MIBC): a multicenter phase 2 trial. J Clin Oncol. 2021;39(15_suppl):4504.
Garcia del Muro X, Valderrama BP, Medina A, Cuellar MA, Etxaniz O, Gironés Sarrió R, et al. Phase II trial of durvalumab plus tremelimumab with concurrent radiotherapy (RT) in patients (pts) with localized muscle invasive bladder cancer (MIBC) treated with a selective bladder preservation approach: IMMUNOPRESERVE-SOGUG trial. J Clin Oncol. 2021;39(15_suppl):4505.
Galsky MD, Daneshmand S, Chan KG, Dorff TB, Cetnar JP, Neil OB, et al. Phase 2 trial of gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle-invasive bladder cancer (MIBC): HCRN GU 16-257. J Clin Oncol. 2021;39(15_suppl):4503.
Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Mayrhofer and D. Niedersüß-Beke declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mayrhofer, K., Niedersüß-Beke, D. Best of ASCO 2021—bladder cancer. memo 14, 335–337 (2021). https://doi.org/10.1007/s12254-021-00765-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-021-00765-7