Abstract
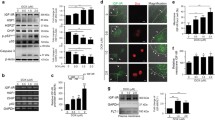
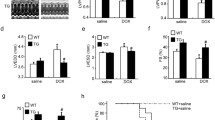
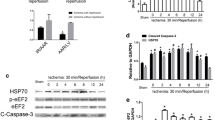
Heat shock proteins (HSPs) play important roles in cellular stress resistance. Previous reports had already suggested that HSP27 played multiple roles in preventing doxorubicin-induced cardiotoxicity. Although HSP25 might have biological functions similar to its human homolog HSP27, the mechanism of HSP25 is still unclear in doxorubicin-induced cardiomyocyte apoptosis. To investigate HSP25 biological function on doxorubicin-induced apoptosis, flow cytometry was employed to analyze cell apoptosis in over-expressing HSP25 H9c2 cells in presence of doxorubicin. Unexpectedly, the H9c2 cells of over-expressing HSP25 have no protective effect on doxorubicin-induced apoptosis. Moreover, no detectable interactions were detected by coimmunoprecipitation between HSP25 and cytochrome c, and HSP25 over-expression failed in preventing cytochrome c release induced by doxorubicin. However, down-regulation of endogenous HSP25 by a specific small hairpin RNA aggravates apoptosis in H9c2 cells. Subsequent studies found that HSP25, but not HSP90, HSP70, and HSP20, interacted with SIRT1. Knockdown of HSP25 decreased the interaction between SIRT1 and p53, leading to increased p53 acetylation on K379, up-regulated pro-apoptotic Bax protein expression, induced cytochrome c release, and triggered caspase-3 and caspase-9 activation. These findings indicated a novel mechanism by which HSP25 regulated p53 acetylation through dissociation of SIRT1 from p53 in doxorubicin-induced H9c2 cell apoptosis.





Similar content being viewed by others
Abbreviations
- shRNA:
-
Small hairpin RNA
- cDNA:
-
Complementary DNA
- RNAi:
-
RNA interference
- FITC:
-
Fluorescein isothiocyanate
- HSPs:
-
Heat shock proteins
- HSP25:
-
Heat shock protein 25
- HSP27:
-
Heat shock protein 27
- SIRT1:
-
Sirtuin 1
- DOX:
-
Doxorubicin
- GAPDH:
-
Glycolytic glyceraldehyde-3-phosphate dehydrogenase
- VDAC:
-
Voltage-dependent anion channel
- TBST:
-
Tris-buffered saline Tween
- BSA:
-
Bovine serum albumin
- FCS:
-
Fetal calf serum
- DMEM:
-
Dulbecco’s modified Eagle’s medium
References
Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2:645–652
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31:164–172
Charette SJ, Landry J (2000) The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in Fas-induced apoptosis. Ann N Y Acad Sci 926:126–131
Chen D, Pacal M, Wenzel P, Knoepfler PS, Leone G, Bremner R (2009) Division and apoptosis of E2f-deficient retinal progenitors. Nature 462:925–929
Chua CC, Liu X, Gao J, Hamdy RC, Chua BH (2006) Multiple actions of pifithrin-alpha on doxorubicin-induced apoptosis in rat myoblastic H9c2 cells. Am J Physiol Heart Circ Physiol 290:H2606–2613
Ciocca DR, Fuqua SA, Lock-Lim S, Toft DO, Welch WJ, McGuire WL (1992) Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res 52:3648–3654
Clarke JP, Mearow KM (2013) Cell stress promotes the association of phosphorylated HspB1 with F-actin. PLoS One 8:e68978
Concannon CG, Gorman AM, Samali A (2003) On the role of Hsp27 in regulating apoptosis. Apoptosis 8:61–70
Feng Y, Zhang C, Luo Q, Wei X, Jiang B, Zhu H, Zhang L, Jiang L, Liu M, Xiao X (2012) A novel WD-repeat protein, WDR26, inhibits apoptosis of cardiomyocytes induced by oxidative stress. Free Radic Res 46:777–784
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C (2003) PI3K/Akt and apoptosis: size matters. Oncogene 22:8983–8998
Goldbaum O, Riedel M, Stahnke T, Richter-Landsberg C (2009) The small heat shock protein HSP25 protects astrocytes against stress induced by proteasomal inhibition. Glia 57:1566–1577
Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, Schwartz JH, Borkan SC (2008) Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem 283:12305–12313
Jin HJ, Xie XL, Ye JM, Li CG (2013) TanshinoneIIA and cryptotanshinone protect against hypoxia-induced mitochondrial apoptosis in H9c2 cells. PLoS One 8:e51720
Kim JH, Yoon EK, Chung HJ, Park SY, Hong KM, Lee CH, Lee YS, Choi K, Yang Y, Kim K, Kim IH (2013) p53 acetylation enhances Taxol-induced apoptosis in human cancer cells. Apoptosis 18:110–120
Krishnamurthy K, Kanagasabai R, Druhan LJ, Ilangovan G (2012) Heat shock protein 25-enriched plasma transfusion preconditions the heart against doxorubicin-induced dilated cardiomyopathy in mice. J Pharmacol Exp Ther 341:829–839
Lee YJ, Lee DH, Cho CK, Bae S, Jhon GJ, Lee SJ, Soh JW, Lee YS (2005) HSP25 inhibits protein kinase C delta-mediated cell death through direct interaction. J Biol Chem 280:18108–18119
Lee HJ, Kwon HC, Chung HY, Lee YJ, Lee YS (2012a) Recovery from radiation-induced bone marrow damage by HSP25 through Tie2 signaling. Int J Radiat Oncol Biol Phys 84:e85–93
Lee YJ, Lee HJ, Choi SH, Jin YB, An HJ, Kang JH, Yoon SS, Lee YS (2012b) Soluble HSPB1 regulates VEGF-mediated angiogenesis through their direct interaction. Angiogenesis 15:229–242
Lin HH, Chen YH, Chiang MT, Huang PL, Chau LY (2013) Activator protein-2alpha mediates carbon monoxide-induced stromal cell-derived factor-1alpha expression and vascularization in ischemic heart. Arterioscler Thromb Vasc Biol 33:785–794
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W (2001) Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107:137–148
Ma J, Wang Y, Zheng D, Wei M, Xu H, Peng T (2013) Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc Res 97:77–87
Mounier N, Arrigo AP (2002) Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 7:167–176
Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E (2000) Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: in vivo study. Circulation 102:572–578
Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP (2002) Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol 22:816–834
Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB (2003) Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 278:27828–27835
Rokudai S, Laptenko O, Arnal SM, Taya Y, Kitabayashi I, Prives C (2013) MOZ increases p53 acetylation and premature senescence through its complex formation with PML. Proc Natl Acad Sci U S A 110:3895–3900
Takata T, Ishikawa F (2003) Human Sir2-related protein SIRT1 associates with the bHLH repressors HES1 and HEY2 and is involved in HES1- and HEY2-mediated transcriptional repression. Biochem Biophys Res Commun 301:250–257
Turakhia S, Venkatakrishnan CD, Dunsmore K, Wong H, Kuppusamy P, Zweier JL, Ilangovan G (2007) Doxorubicin-induced cardiotoxicity: direct correlation of cardiac fibroblast and H9c2 cell survival and aconitase activity with heat shock protein 27. Am J Physiol Heart Circ Physiol 293:H3111–3121
Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR (1998) Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer J 79:468–475
Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107:149–159
Venkatakrishnan CD, Tewari AK, Moldovan L, Cardounel AJ, Zweier JL, Kuppusamy P, Ilangovan G (2006) Heat shock protects cardiac cells from doxorubicin-induced toxicity by activating p38 MAPK and phosphorylation of small heat shock protein 27. Am J Physiol Heart Circ Physiol 291:H2680–2691
Venkatakrishnan CD, Dunsmore K, Wong H, Roy S, Sen CK, Wani A, Zweier JL, Ilangovan G (2008) HSP27 regulates p53 transcriptional activity in doxorubicin-treated fibroblasts and cardiac H9c2 cells: p21 upregulation and G2/M phase cell cycle arrest. Am J Physiol Heart Circ Physiol 294:H1736–1744
Wang K, Lei J, Zou J, Xiao H, Chen A, Liu X, Liu Y, Jiang L, Xiao Z, Xiao X (2013) Mipu1, a novel direct target gene, is involved in hypoxia inducible factor 1-mediated cytoprotection. PLoS One 8:e82827
Yamamoto H, Schoonjans K, Auwerx J (2007) Sirtuin functions in health and disease. Mol Endocrinol 21:1745–1755
Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, Liu M, Chen G, Xiao X (2011) Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res 90:538–545
Acknowledgments
This work was supported by grants from the Major National Basic Research Program of China (no. 2007CB512007), the National Natural Science Foundation of China (81100106; 81100212; 81300113), A Project Supported by Scientific Research Fund of Hunan Provincial Education Department (11C1094; 11C1095), and the National College Students’ Innovation and Entrepreneurship Training Program (201210555011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, C., Qu, S., Wei, X. et al. HSP25 down-regulation enhanced p53 acetylation by dissociation of SIRT1 from p53 in doxorubicin-induced H9c2 cell apoptosis. Cell Stress and Chaperones 21, 251–260 (2016). https://doi.org/10.1007/s12192-015-0655-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-015-0655-3