Abstract
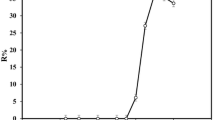
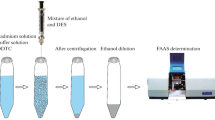
A new, simple, and ligandless ion-pair microextraction method has been developed for preconcentration and determination of cadmium ion in saline solutions and food samples. This technique is based on using a double chain cationic surfactant called didodecyldimethylammonium bromide (DDAB) as an ion-pairing agent and extraction solvent combined with dispersive microextraction named supramolecule aggregate-based dispersive liquid-solid microextraction (SA-DLSME) prior to aspiration to flame atomic absorption spectrometer. The Cd2+ ion interacts with chloride ion in saline solution, and the resulting anionic complex is microextracted by cationic supramolecule aggregates of DDAB due to electrostatic interaction. This technique benefits the advantages of supramolecule aggregate properties and dispersive liquid-liquid microextraction technique without the use of any dispersing solvent. Phase separation behavior of DDAB and several effective parameters that have influence on extraction efficiency of cadmium ion such as pH, salt concentration, centrifugation time, and DDAB amount were thoroughly investigated and optimized. Under the optimized experimental conditions, the limit of detection (LOD) and linear range were 1.3 and 5–250 ng mL−1, respectively, with relative standard deviations (RSD) in the range of 3.1–4.3 for interday analysis and 4.5–5.5 for intraday tests at different concentrations of cadmium. The proposed method was successfully applied for the determination of cadmium ion in real samples, and its accuracy was confirmed by analysis of a certified reference material.






Similar content being viewed by others
References
Abadi MDM, Chamsaz M, Arbab-Zavar MH, Shemirani F (2013) Supramolecular dispersive liquid–liquid microextraction based solidification of floating organic drops for speciation and spectrophotometric determination of chromium in real samples. Anal Methods 5:2971–2977
Agency for Toxic Substances and Disease Registry (ATSDR) (1998) Toxicological profile for cadmium. U.S. Department of Health and Human Services, Public Health Service, U.S. Government Printing Office, USA
Anthemidis AN, Ioannou K-IG (2009) Recent developments in homogeneous and dispersive liquid–liquid extraction for inorganic elements determination. A review. Talanta 80:413–421
Baliza PX, Cardoso LAM, Lemos VA (2012) A preconcentration procedure for the determination of cadmium in biological material after on-line cloud point extraction. Environ Monit Assess 184:4455–4460
Ballesteros-Gómez A, Sicilia MD, Rubio S (2010) Supramolecular solvents in the extraction of organic compounds. A review. Anal Chim Acta 677:108–130
Bianchin JN, Martendal E, Mior R, Alves VN, Araújo CST, Coelho NMM, Carasek E (2009) Development of a flow system for the determination of cadmium in fuel alcohol using vermicompost as biosorbent and flame atomic absorption spectrometry. Talanta 78:333–336
Caballo C, Costi EM, Sicilia MD, Rubio S (2012) Determination of supplemental feeding needs for astaxanthin and canthaxanthin in salmonids by supramolecular solvent-based microextraction and liquid chromatography–UV/VIS spectroscopy. Food Chem 134:1244–1249
Cerutti S, Silva M, Gasquez J, Olsina R, Martinez L (2003) On-line preconcentration/determination of cadmium in drinking water on activated carbon using 8-hydroxyquinoline in a flow injection system coupled to an inductively coupled plasma optical emission spectrometer. Spectrochim Acta B 58:43–50
Chamsaz M, Eftekhari M, Atarodi A, Asadpour S, Ariani M (2012) Preconcentration procedure using vortex agitator system for determination of trace levels of cadmium by flame atomic absorption spectrometry. J Braz Chem Soc 23:1630–1635
D’Ilio S, Petrucci F, D’Amato M, Di Gregorio M, Senofonte O, Violante N (2008) Method validation for determination of arsenic, cadmium, chromium and lead in milk by means of dynamic reaction cell inductively coupled plasma mass spectrometry. Anal Chim Acta 624:59–67
Fang G-Z, Tan J, Yan X-P (2005) An ion-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique combined with a sol-gel process for selective solid-phase extraction of cadmium (II). Anal Chem 77:1734–1739
Jafarvand S, Shemirani F (2011a) Supramolecular‐based dispersive liquid–liquid microextraction: a novel sample preparation technique utilizes coacervates and reverse micelles. J Sep Sci 34:455–461
Jafarvand S, Shemirani F (2011b) Supramolecular-based dispersive liquid–liquid microextraction: a novel sample preparation technique for determination of inorganic species. Microchim Acta 173:353–359
Jafarvand S, Shemirani F (2011c) Supramolecular-based dispersive liquid–liquid microextraction: determination of cadmium in water and vegetable samples. Analytical Methods 3:1552–1559
Jia G, Li L, Qiu J, Wang X, Zhu W, Sun Y, Zhou Z (2007) Determination of carbaryl and its metabolite 1-naphthol in water samples by fluorescence spectrophotometer after anionic surfactant micelle-mediated extraction with sodium dodecylsulfate. Spectrochim Acta A Mol Biomol Spectrosc 67:460–464
Jin X, Zhu M, Conte ED (1999) Surfactant-mediated extraction technique using alkyltrimethylammonium surfactants: extraction of selected chlorophenols from river water. Anal Chem 71:514–517
Komjarova I, Blust R (2006) Comparison of liquid–liquid extraction, solid-phase extraction and co-precipitation preconcentration methods for the determination of cadmium, copper, nickel, lead and zinc in seawater. Anal Chim Acta 576:221–228
Kumar S, Khan ZA (2004) Clouding phenomenon in ionic micellar solutions: role of the counterion. J Surfactant Deterg 7:367–371
Kwok-Wai Man B, Hon-Wah Lam M, Lam PK, Wu RS, Shaw G (2002) Cloud-point extraction and preconcentration of cyanobacterial toxins (microcystins) from natural waters using a cationic surfactant. Environ Sci Technol 36:3985–3990
Luciano RM, Bedendo GC, Carletto JS, Carasek E (2010) Isolation and preconcentration of Cd (II) from environmental samples using polypropylene porous membrane in a hollow fiber renewal liquid membrane extraction procedure and determination by FAAS. J Hazard Mater 177:567–572
Ma JJ, Du X, Zhang JW, Li JC, Wang LZ (2009) Ultrasound-assisted emulsification–microextraction combined with flame atomic absorption spectrometry for determination of trace cadmium in water samples. Talanta 80:980–984
Marques E, Regev O, Khan A, Lindman B (2003) Self-organization of double-chained and pseudodouble-chained surfactants: counterion and geometry effects. Adv Colloid Interf Sci 100:83–104
Monticelli D, Ciceri E, Dossi C (2007) Optimization and validation of an automated voltammetric stripping technique for ultratrace metal analysis. Anal Chim Acta 594:192–198
Moradi M, Yamini Y (2012) Application of vesicular coacervate phase for microextraction based on solidification of floating drop. J Chromatogr A 1229:30–37
Nan Y, Liu H, Hu Y (2005) Aqueous two-phase systems of cetyltrimethylammonium bromide and sodium dodecyl sulfonate mixtures without and with potassium chloride added. Colloids Surf A Physicochem Eng Asp 269:101–111
Regev O, Khan A (1994) Vesicle–lamellar transition events in DDAB-water solution. Progr Colloid Polym Sci 97:298–301
Rezaee M, Assadi Y, Hosseini M-RM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9
Rivas RE, López-García I, Hernández-Córdoba M (2009) Determination of traces of lead and cadmium using dispersive liquid-liquid microextraction followed by electrothermal atomic absorption spectrometry. Microchim Acta 166:355–361
Rojas FS, Ojeda CB, Pavon JC (2011) Dispersive liquid–liquid microextraction combined with flame atomic absorption spectrometry for determination of cadmium in environmental, water and food samples. Anal Methods 3:1652–1655
Sarafraz-Yazdi A, Amiri A (2010) Liquid-phase microextraction. TrAC Trends Anal Chem 29:1–14
Sarafraz Yazdi A (2011) Surfactant-based extraction methods. TrAC Trends Anal Chem 30:918–929
Saryan LA, Zenz C (1994) In: Zenz OC, Dickerson B, Horvath EP (eds) Occupational medicine, 3rd edn. Mosby-Year Book, USA, p 481
Warr GG, Sen R, Evans DF, Trend JE (1988) Microemulsion formation and phase behavior of dialkydimethylammonium bromide surfactants. J Phys Chem 92:774–783
Watanabe H, Tanaka H (1978) A non-ionic surfactant as a new solvent for liquid–liquid extraction of zinc (II) with 1-(2-pyridylazo)-2-naphthol. Talanta 25:585–589
Xie F, Lin X, Wu X, Xie Z (2008) Solid phase extraction of lead (II), copper (II), cadmium (II) and nickel (II) using gallic acid-modified silica gel prior to determination by flame atomic absorption spectrometry. Talanta 74:836–843
Zgoła-Grześkowiak A, Grześkowiak T (2011) Dispersive liquid-liquid microextraction. TrAC Trends Anal Chem 30:1382–1399
Zhou Q, Bai H, Xie G, Xiao J (2008) Temperature-controlled ionic liquid dispersive liquid phase micro-extraction. J Chromatogr A 1177:43–49
Acknowledgments
The authors would like to thank Ferdowsi University of Mashhad for financial support of this work.
Funding
This study was funded by Ferdowsi University of Mashhad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Hadi Kahe, Mahmoud Chamsaz, and Gholam Hossein Rounaghi declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kahe, H., Chamsaz, M. & Rounaghi, G.H. A Microextraction Method Based on Ligandless Ion-Pair Formation for Measuring the Cadmium Cation in Real Samples by Flame Atomic Absorption Spectrometry. Food Anal. Methods 9, 2887–2895 (2016). https://doi.org/10.1007/s12161-016-0484-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0484-8