Abstract
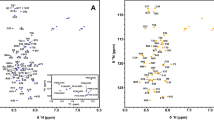
Tensin is an important cytoplasmic phosphoprotein localized to integrin-mediated focal adhesion. It links actin cytoskeleton to extracellular matrix through its N-terminal actin-binding domain and C-terminal phosphotyrosine-binding domain. Studies of knockout mice revealed the critical roles of tensin in skeletal muscle regeneration, renal function and regulation of cell migration. The SH2 domain of tensin interacts with various tyrosine-phosphorylated proteins thus functions as a platform for dis/assembly of signaling molecules. It has also been implicated in recruiting a tumor supperssor protein DLC1 (deleted in live cancer 1) to the focal adhesion, which is required for oncogenic inhibition effect of DLC1 in a phosphotyrosine-independent manner. Here, we report complete chemical shift assignments of the SH2 domain of human tensin2 determined by triple resonance experiments. The resonance assignments serve as a basis for our further functional studies and structure determination by NMR spectroscopy. (BMRB deposits with accession number 16472).



Similar content being viewed by others
References
Auger KR, Songyang Z, Lo SH, Roberts TM, Chen LB (1996) Platelet-derived growth factor-induced formation of tensin and phosphoinositide 3-kinase complexes. J Biol Chem 271:23452–23457
Bax A, Grzesiek S (1993) Methodological advances in protein NMR. Acc Chem Res 26:131–138
Chen H, Lo SH (2003) Regulation of tensin-promoted cell migration by its focal adhesion localization and the SH2 domain. Biochem J 370:1039–1045
Clore GM, Gronenborn AM (1998) Determining structures of large proteins and protein complexes by NMR. Trends Biotechnol 16:22–34
Davis S, Lu ML, Lo SH, Lin S, Butter J, Druker B, Roberts T, An Q, Chen LB (1991) Presence of an SH2 domain in the actin-binding protein tensin. Science 252:712–715
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Ishii A, Lo SH (2001) A role of tensin in skeletal-muscle regeneration. Biochem J 356:737–745
Liao YC, Si L, deVere White RW, Lo SH (2007) The phosphotyrosineindependent interaction of DLC-1 and the SH2 domain of cten regulates focal adhesion localization and growth suppression activity of DLC-1. J Cell Biol 176:43–49
Lo SH (2004) Molecules in focus: tensin. Int J Biochem Cell Biol 36:31–34
Lo SH, An Q, Bao S, Wong W, Liu Y, Janmey PA, Hartwig JA, Chen LB (1994) Molecular cloning of chick cardiac muscle tensin, full-length cDNA sequence, expression, and characterization. J Biol Chem 269:22310–22319
Lo SH, Yu QC, Degenstein L, Chen LB, Fuchs E (1997) Progressive kidney degeneration in mice lacking tensin. J Cell Biol 136:1349–1361
Qian X, Li G, Asmussen HK, Asnaghi L, Vass WC et al (2007) Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc Natl Acad Sci USA 104:9012–9017
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectrosc 34:93–158
Wishart DS, Sykes BD (1994) The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR 4:171–180
Zhu G, Bax A (1992) Improved linear prediction of truncated damped sinusoids using modified backward-forward linear prediction. J Magn Reson 100:202–207
Acknowledgments
The work described in this paper was supported by a grant from Hong Kong Research Grants Council (RGC664109 and TUYF10SC03 to G.Z). We thank Mr. Feng Rui for helping to set up the NMR experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Liu, C., Rui, F. et al. 1H, 15N and 13C chemical shift assignments of the SH2 domain of human tensin2 (TENC1). Biomol NMR Assign 5, 211–214 (2011). https://doi.org/10.1007/s12104-011-9302-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-011-9302-9