Abstract
Objectives
Plasmablastic lymphoma (PBL) is a subtype of diffuse large B-cell lymphoma (DLBCL) often associated with Epstein–Barr virus (EBV) infection. Despite recent advances in treatment, PBL still has a poor prognosis. EBV is listed as one of the human tumor viruses that may cause cancer, and is closely related to the occurrence of some nasopharyngeal carcinoma (NPC), lymphoma and 10% of gastric cancer (GC). It is very important to explore the differentially expressed genes (DEGs) between EBV-positive and EBV-negative PBL. Through bioinformatics analysis of DEGs between EBV-positive PBL and EBV-negative PBL, we gain a deeper understanding of the pathogenesis of EBV-positive PBL.
Methods
We selected the GSE102203 data set, and screened the DEGs between EBV-positive PBL and EBV-negative PBL. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were applied. The protein–protein interaction (PPI) network was constructed, and screened for the hub genes. Finally, Gene Set Enrichment Analysis (GSEA) was performed.
Results
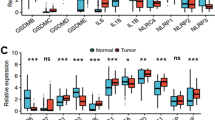
In EBV-positive PBL, the immune-related pathway is upregulated and Cluster of differentiation 27 (CD27) and programmed cell death-ligand 1 (PD-L1) are hub genes.
Conclusions
In EBV-positive PBL, EBV may affect tumorigenesis through activation of immune-related pathways and upregulation of CD27, PD-L1. Immune checkpoint blockers of CD70/CD27 and programmed cell death 1 (PD-1)/PD-L1 pathways may be one of the effective strategies for the treatment of EBV-positive PBL.




Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- PBL:
-
Plasmablastic lymphoma
- DLBCL:
-
Diffuse large B cell lymphoma
- EBV:
-
Epstein–Barr virus
- NPC:
-
Nasopharyngeal carcinoma
- GC:
-
Gastric cancer
- DEGs:
-
Differentially expressed genes
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- PPI:
-
Protein–protein interaction
- GSEA:
-
Gene set enrichment analysis
- CD27:
-
Cluster of differentiation
- PD-L1:
-
Programmed cell death-ligand 1
- PD-1:
-
Programmed cell death 1
- ISH:
-
In situ hybridization
- HIV:
-
Human immunodeficiency virus
- CC:
-
Cellular component
- MF:
-
Molecular function
- BP:
-
Biological process
- Th1:
-
T-helper type 1
- ARLs:
-
AIDS-related lymphomas
- EBNA1:
-
Epstein–Barr nuclear antigen 1
- EBERs:
-
EBV-encoded RNAs
- TNFRSF:
-
Tumor necrosis factor receptor superfamily
- RCC:
-
Renal cell carcinoma
- LMP2A:
-
Latent membrane protein 2A
- ICIs:
-
Immune checkpoint inhibitors
- HPD:
-
Hyperprogressive disease
- NSCLC:
-
Non-small cell lung cancer
- HNSCC:
-
Head and neck squamous cell carcinoma
References
Khan G, Fitzmaurice C, Naghavi M, Ahmed LA. Global and regional incidence, mortality and disability-adjusted life-years for Epstein-Barr virus-attributable malignancies, 1990–2017. BMJ Open. 2020;10:e037505. https://doi.org/10.1136/bmjopen-2020-037505.
Bhatt R, Desai DS. StatPearls. Treasure Island, FL: Publishing Copyright © 2021, StatPearls Publishing LLC; 2021.
Fernández-Álvarez R, Sancho JM, Ribera JM. Plasmablastic lymphoma. Med Clin. 2016;147:399–404. https://doi.org/10.1016/j.medcli.2016.06.036.
Han B, Feng D, Yu X, Zhang Y, Liu Y, Zhou L. Identification and interaction analysis of molecular markers in colorectal cancer by integrated bioinformatics analysis. Med Sci Monit. 2018;24:6059–69. https://doi.org/10.12659/msm.910106.
Liu J, Li R, Liao X, Jiang W. Comprehensive bioinformatic analysis genes associated to the prognosis of liposarcoma. Med Sci Monit. 2018;24:7329–39. https://doi.org/10.12659/msm.913043.
Da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. https://doi.org/10.1038/nprot.2008.211.
Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;15:2323–30. https://doi.org/10.1182/blood-2014-10-567479.
Bibas M, Castillo JJ. Current knowledge on HIV-associated plasmablastic lymphoma. Mediterr J Hematol Infect Dis. 2014;6:e2014064. https://doi.org/10.4084/MJHID.2014.064.
Carbone A, Vaccher E, Gloghini A, Pantanowitz L, Abayomi A, de Paoli P, et al. Diagnosis and management of lymphomas and other cancers in HIV-infected patients. Nat Rev Clin Oncol. 2014;11:223–38. https://doi.org/10.1038/nrclinonc.2014.31.
Castillo JJ, Furman M, Beltrán BE, Bibas M, Bower M, Chen W, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer. 2012;21:5270–7. https://doi.org/10.1002/cncr.27551.
Hintzen RQ, de Jong R, Lens SM, van Lier RA. CD27: marker and mediator of T cell activation? Immunol Today. 1994;15:307–11. https://doi.org/10.1016/0167-5699(94)90077-9.
Yu L, Li L, Medeiros LJ, Young KH. NF-κB signaling pathway and its potential as a target for therapy in lymphoid neoplasms. Blood Rev. 2017;31:77–92. https://doi.org/10.1016/j.blre.2016.10.001.
Barnabei L, Laplantine E, Mbongo W, Rieux-Laucat F, Weil R. NF-κB: at the borders of autoimmunity and inflammation. Front Immunol. 2021;12:716469. https://doi.org/10.3389/fimmu.2021.716469.
Hövelmeyer N, Schmidt-Supprian M, Ohnmacht C. NF-κB in control of regulatory T cell development, identity, and function. J Mol Med (Berl). 2022;100:985–95. https://doi.org/10.1007/s00109-022-02215-1.
Cai MB, Han HQ, Bei JX, Liu CC, Lei JJu, Cui Q, et al. Expression of human leukocyte antigen G is associated with prognosis in nasopharyngeal carcinoma. Int J Biol Sci. 2012;8:891–900. https://doi.org/10.7150/ijbs.4383.
Morales O, Mrizak D, François V, Mustapha R, Miroux C, Depil S, et al. Epstein–Barr virus infection induces an increase of T regulatory type 1 cells in Hodgkin lymphoma patients. Br J Haematol. 2014;166:875–90. https://doi.org/10.1111/bjh.12980.
Sun SC, Cesarman E. NF-κB as a target for oncogenic viruses. Curr Top Microbiol Immunol. 2011;349:197–244. https://doi.org/10.1007/82_2010_108.
Starzer AM, Berghoff AS. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open Suppl. 2020;3:e000629. https://doi.org/10.1136/esmoopen-2019-000629.
Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33:769–79. https://doi.org/10.1097/CJI.0b013e3181ee238f.
Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12:92. https://doi.org/10.1186/s13045-019-0779-5.
Li X, Zhang W. Expression of PD-L1 in EBV-associated malignancies. Int Immuno pharmacol. 2021;95:107553. https://doi.org/10.1016/j.intimp.2021.107553.
Gru AA, Haverkos BH, Freud AG, Hastings J, Nowacki NB, Barrionuevo C, et al. The Epstein–Barr virus (EBV) in T Cell and NK cell lymphomas: time for a reassessment. Curr Hematol Malig Rep. 2015;10:456–67. https://doi.org/10.1007/s11899-015-0292-z.
Granai M, Mundo L, Akarca AU, Siciliano MC, Rizvi H, Mancini V, et al. Immune landscape in Burkitt lymphoma reveals M2-macrophage polarization and correlation between PD-L1 expression and non-canonical EBV latency program [published correction appears in infect agent cancer. Infect Agent Cancer. 2020;15:28. https://doi.org/10.1186/s13027-020-00292-w.
Rosado FG, Coberly J, Gupta A, John G, Naina H, Koduru P, et al. PD1/PD-L1 expressions in plasmablastic lymphoma with clinicopathological correlation. Ann Clin Lab Sci. 2021;51:174–81.
Ahrends T, Bąbała N, Xiao Y, Yagita H, van Eenennaam H, Borst J. CD27 Agonism Plus PD-1 blockade recapitulates CD4+ T-cell help in therapeutic anticancer vaccination. Cancer Res. 2016;76:2921–31. https://doi.org/10.1158/0008-5472.CAN-15-3130.
Buchan SL, Fallatah M, Thirdborough SM, Taraban VY, Rogel A, Thomas LJ, et al. PD-1 blockade and CD27 stimulation activate distinct transcriptional programs that synergize for CD8+ T cell-driven antitumor immunity. Clin Cancer Res. 2018;24:2383–94. https://doi.org/10.1158/1078-0432.CCR-17-3057.
Kim JY, Lee KH, Kang J, Borcoman E, Saada-Bouzid E, Kronbichler A, et al. Hyperprogressive disease during anti-PD-1 (PDCD1)/PD-L1 (CD274) therapy: a systematic review and meta-analysis. Cancers (Basel). 2019;11:1699. https://doi.org/10.3390/cancers11111699.
Acknowledgements
Yue Liang, Hanqing Wang, Bing Luo designed research; Yue Liang, Hanqing Wang analyzed data; Yue Liang performed research and wrote the paper; Yue Liang, Bing Luo agreed to be accountable for all aspects of the work. Thanks to everyone who helped with this article. The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Y., Wang, H. & Luo, B. Exploration and analysis of differentially expressed genes in Epstein–Barr virus negative and positive plasmablastic lymphoma. Clin Transl Oncol 25, 2884–2891 (2023). https://doi.org/10.1007/s12094-023-03150-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03150-4