Abstract
Purpose
The aim of this study was to investigate the involvement of the SDF-1/CXCR4 axis in the process of BMMSC homing in prostate cancer (PCa) in vivo and in vitro.
Methods
After verification of BMMSCs, we fixed the concentration gradient of SDF-1 for BMMSC cultivation to analyze CXCR4 expression by qRT-PCR and flow cytometric analysis. Furthermore, we developed a non-contact co-culture system and explored the participation of the SDF-1/CXCR4 axis in PCa using qRT-PCR, flow cytometry, and ELISA. In addition, A green fluorescent protein (GFP)-transplanted methylnitrosourea (MNU)-induced PCa mouse model was established to investigate the CXCR4 expression in vivo.
Results
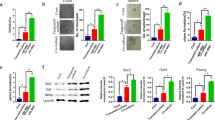
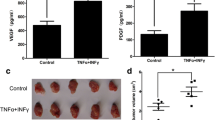
The CXCR4 expression was up-regulated with the increase in SDF-1 concentrations, and elevated SDF-1 had a significant promoting effect on cell proliferation and migration in BMMSCs. Moreover, the CXCR4 expression of BMMSCs was significantly increased in the non-contact co-culture model with vascular endothelial cells (VECs), and analysis of this model also showed that the proliferation and migration of BMMSCs were promoted in the presence of VECs. The ELISA assay showed that the SDF-1 levels in the co-culture model at 48 h were significantly increased. Twenty of the GFP-transplanted mice were divided into a PCa group and a control group, and four GFP-transplanted mice were observed to have prostate tumorigenesis. It also showed that CXCR4 was obviously increased in the prostate tissue of PCa mice.
Conclusion
Our findings suggest that BMMSCs could home and promote the proliferation and migration of PCa through the SDF-1/CXCR4 axis in vivo and in vitro.




Similar content being viewed by others
Code availability
Not applicable.
Abbreviations
- PCa:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- RP:
-
Radical prostatectomy
- ADT:
-
Androgen deprivation therapy
- AR:
-
Androgen receptor
- BMDCs:
-
Bone marrow-derived cells
- BMMSCs:
-
Bone marrow mesenchymal stem cells
- GFP:
-
Green fluorescent protein
- TRAMP:
-
Transplanted transgenic adenocarcinoma of mouse prostate
- MUN:
-
Methylnitrosourea
- SDF-1:
-
Stromal cell-derived factor-1
- VECs:
-
Vascular endothelial cells
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
Perdana NR, Mochtar CA, Umbas R, Hamid ARA. The risk factors of prostate cancer and its prevention: a literature review. Acta Med Indones. 2016;48(3):228–38.
Daniyal M, Siddiqui ZA, Akram M, Asif HM, Sultana S, Khan A. Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac J Cancer Prev. 2014;15(22):9575–8. https://doi.org/10.7314/apjcp.2014.15.22.9575.
Tolkach Y, Kristiansen G. The heterogeneity of prostate cancer: a practical approach. Pathobiology. 2018;85(1–2):108–16. https://doi.org/10.1159/000477852.
Ciccarese C, Massari F, Iacovelli R, Fiorentino M, Montironi R, Di Nunno V, et al. Prostate cancer heterogeneity: discovering novel molecular targets for therapy. Cancer Treat Rev. 2017;54:68–73. https://doi.org/10.1016/j.ctrv.2017.02.001.
Neschadim A, Summerlee AJS, Silvertown JD. Targeting the relaxin hormonal pathway in prostate cancer. Int J Cancer. 2015;137(10):2287–95. https://doi.org/10.1002/ijc.29079.
Stone L. Prostate cancer: the pathway to progression: DHT biosynthesis. Nat Rev Urol. 2017;14(9):516. https://doi.org/10.1038/nrurol.2017.118.
Schneider JA, Logan SK. Revisiting the role of Wnt/β-catenin signaling in prostate cancer. Mol Cell Endocrinol. 2018;462(Pt A):3–8. https://doi.org/10.1016/j.mce.2017.02.008.
Patel M, Sachidanandan M, Adnan M. Serine arginine protein kinase 1 (SRPK1): a moonlighting protein with theranostic ability in cancer prevention. Mol Biol Rep. 2019;46(1):1487–97. https://doi.org/10.1007/s11033-018-4545-5.
Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306(5701):1568–71. https://doi.org/10.1126/science.1099513.
Yan X, Zhang D, Wu W, Wu S, Qian J, Hao Y, et al. Mesenchymal stem cells promote hepatocarcinogenesis via lncRNA-MUF interaction with ANXA2 and miR-34a. Can Res. 2017;77(23):6704–16. https://doi.org/10.1158/0008-5472.CAN-17-1915.
Pietrovito L, Leo A, Gori V, Lulli M, Parri M, Becherucci V, et al. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol Oncol. 2018;12(5):659–76. https://doi.org/10.1002/1878-0261.12189.
Zhou X-M, Wang D, He H-L, Tang J, Wu J, Xu L, et al. Bone marrow derived mesenchymal stem cells involve in the lymphangiogenesis of lung cancer and Jinfukang inhibits the involvement in vivo. J Cancer. 2017;8(10):1786–94. https://doi.org/10.7150/jca.17859.
Liu X-Z, Zhang L-T, Tong W-Q, Wang S, Xu Z-B, Chen F. Research advances in molecular mechanisms of the invasion and metastasis of lung cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38(1):108–12. https://doi.org/10.3881/j.issn.1000-503X.2016.01.020.
Luo F, Liu T, Wang J, Li J, Ma P, Ding H, et al. Bone marrow mesenchymal stem cells participate in prostate carcinogenesis and promote growth of prostate cancer by cell fusion in vivo. Oncotarget. 2016;7(21):30924–34. https://doi.org/10.18632/oncotarget.9045.
Wen S, Niu Y, Yeh S, Chang C. BM-MSCs promote prostate cancer progression via the conversion of normal fibroblasts to cancer-associated fibroblasts. Int J Oncol. 2015;47(2):719–27. https://doi.org/10.3892/ijo.2015.3060.
Zhou Y, Cao H-B, Li W-J, Zhao L. The CXCL12 (SDF-1)/CXCR4 chemokine axis: oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin J Nat Med. 2018;16(11):801–10. https://doi.org/10.1016/S1875-5364(18)30122-5.
Teicher BA, Fricker SP. CXCL12(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16(11):2927–31. https://doi.org/10.1158/1078-0432.CCR-09-2329.
Zhou W, Guo S, Liu M, Burow ME, Wang G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr Med Chem. 2019;26(17):3026–41. https://doi.org/10.2174/0929867324666170830111531.
Meng W, Xue S, Chen Y. The role of CXCL12 in tumor microenvironment. Gene. 2018;641:105–10. https://doi.org/10.1016/j.gene.2017.10.015.
Kowalski K, Kołodziejczyk A, Sikorska M, Płaczkiewicz J, Cichosz P, Kowalewska M, et al. Stem cells migration during skeletal muscle regeneration—the role of Sdf-1/Cxcr4 and Sdf-1/Cxcr7 axis. Cell Adhes Migr. 2017;11(4):384–98. https://doi.org/10.1080/19336918.2016.1227911.
Li M, Sun X, Ma L, Jin L, Zhang W, Xiao M, et al. SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3β/β-catenin pathways. Sci Rep. 2017;7:40161. https://doi.org/10.1038/srep40161.
Chen D, Xia Y, Zuo K, Wang Y, Zhang S, Kuang D, et al. Crosstalk between SDF-1/CXCR4 and SDF-1/CXCR7 in cardiac stem cell migration. Sci Rep. 2015;5:16813. https://doi.org/10.1038/srep16813.
Bi J, Li P, Li C, He J, Wang Y, Zhang H, et al. The SDF-1/CXCR4 chemokine axis in uveal melanoma cell proliferation and migration. Tumour Biol. 2016;37(3):4175–82. https://doi.org/10.1007/s13277-015-4259-4.
Chang AI, Schwertschkow AH, Nolta JA, Wu J. Involvement of mesenchymal stem cells in cancer progression and metastases. Curr Cancer Drug Targets. 2015;15(2):88–98. https://doi.org/10.2174/1568009615666150126154151.
Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V. Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med. 2017;6(12):2115–25. https://doi.org/10.1002/sctm.17-0138.
Liu C, Feng X, Wang B, Wang X, Wang C, Yu M, et al. Bone marrow mesenchymal stem cells promote head and neck cancer progression through Periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer Sci. 2018;109(3):688–98. https://doi.org/10.1111/cas.13479.
Mi F, Gong L. Secretion of interleukin-6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma. Biosci Rep. 2017;37(4):BSR20170181. https://doi.org/10.1042/BSR20170181.
Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16(1):31. https://doi.org/10.1186/s12943-017-0597-8.
Luo J, Lee SO, Cui Y, Yang R, Li L, Chang C. Infiltrating bone marrow mesenchymal stem cells (BM-MSCs) increase prostate cancer cell invasion via altering the CCL5/HIF2α/androgen receptor signals. Oncotarget. 2015;6(29):27555–65. https://doi.org/10.18632/oncotarget.4515.
Yang K-Q, Liu Y, Huang Q-H, Mo N, Zhang Q-Y, Meng Q-G, et al. Bone marrow-derived mesenchymal stem cells induced by inflammatory cytokines produce angiogenetic factors and promote prostate cancer growth. BMC Cancer. 2017;17(1):878. https://doi.org/10.1186/s12885-017-3879-z.
Kise K, Kinugasa-Katayama Y, Takakura N. Tumor microenvironment for cancer stem cells. Adv Drug Deliv Rev. 2016;99(Pt B):197–205. https://doi.org/10.1016/j.addr.2015.08.005.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98. https://doi.org/10.1038/nrc.2016.73.
Yu F-X, Hu W-J, He B, Zheng Y-H, Zhang Q-Y, Chen L. Bone marrow mesenchymal stem cells promote osteosarcoma cell proliferation and invasion. World J Surg Oncol. 2015;13:52. https://doi.org/10.1186/s12957-015-0465-1.
Guo Q, Gao B-L, Zhang X-J, Liu G-C, Xu F, Fan Q-Y, et al. CXCL12-CXCR4 axis promotes proliferation, migration, invasion, and metastasis of ovarian cancer. Oncol Res. 2014;22(5–6):247–58. https://doi.org/10.3727/096504015X14343704124430.
Wang C, Cheng H, Li Y. Role of SDF-1 and CXCR4 in the proliferation, migration and invasion of cervical cancer. Pak J Pharm Sci. 2016;29(6 Spec):2151–4.
Funding
This study was supported by Tianjin Health Science and Technology Fund [No. KJ20051 and ZC20182] in collection, analysis, and interpretation of data as well as in writing the manuscript, and Tianjin Union Medical Center Fund [No. 2018YJZD004, 2020HL029, 2020YJ021] in the design of the study and collection, analysis, and interpretation of data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The present study was carried out at Tianjin Union Medical Center and was approved by the Institutional Ethics and Research Committee. The maximum tumor size that was permitted by the ethics committee in the experimental animals used was 15 mm, and we confirmed that the maximum tumor size was not exceeded 15 mm at any point during the duration of study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12094_2021_2740_MOESM1_ESM.tif
Supplementary file1Supplement Fig. 1 A1: Experimental design picture about mouse model. C57BL/6 mice received lethal doses of 137Cs radiation to finish myeloablative, and subjected to retrobulbar injection with about 2 × 106 (100 μl) BMDCs from GFP transgenic mice. Chimera mice were performed an orthotopic injection of MNU (2 mg per mouse) every 3 weeks to induce prostate cancer. A2: Schematic diagram of non-contact model of BMMSCs and VECs cells. B1-4: The separation superstition of BM-MSCS in GFP transgenic mice. C1: Collection of Blood sample of Chimera mice from inner canthus. C2: Castration surgery of Chimera mice. C3-4: MNU injection into mouse prostate (TIF 7160 KB)
Rights and permissions
About this article
Cite this article
Luo, F., Su, Y., Zhang, Z. et al. Bone marrow mesenchymal stem cells promote the progression of prostate cancer through the SDF-1/CXCR4 axis in vivo and vitro. Clin Transl Oncol 24, 892–901 (2022). https://doi.org/10.1007/s12094-021-02740-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02740-4