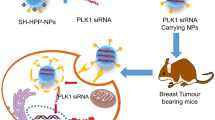
Abstract
Background and objective
To use siRNA molecule as a therapeutic agent in gene silencing, an efficient delivery system is necessary. Stability and clearance by reticuloendothelial of siRNA still remains the major challenges for clinical application. Herein, we could develop new lipid-polymer hybrid nanoparticles (LPHNP) as a siRNA carrier to silence insulin-like growth factor type I (IGF-1R) gene overexpression in MCF-7 human breast cancer cell line.
Methods
Dimethyldioctadecylammonium bromide-methoxy poly(ethylene glycol)-poly (ε-caprolactone) (DDAB-mPEG-PCL) LPHNPs were synthesized using a single step nanoprecipitation method and characterized by dynamic light scattering (DLS) and atomic force microscopy (AFM) microscope. Cytotoxicity of the nanoparticles was assessed in the MCF7 cell line using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Desired LPHNP-siRNA complex was determined using different Nitrogen:Phosphate ratio (N/P) ratios and gel retardation. To determine the encapsulation efficiency of siRNA (%) in LPHNP, its absorbance was measured. The effect of the siRNA-LPHNP complex on IGF-1R silencing was assessed by reverse transcription-polymerase chain reaction (RT-PCR)
Results
LPHNP was synthesized using a single-step sonication method with a size below 100 nM. The viability of cells treated with hybrid nanoparticles was significantly greater than the corresponding cationic lipid (P < 0.01). As demonstrated by gel retardation assay, efficient siRNA binding to LPHNP occurred at N/P equal to 40 and siRNA encapsulation efficiency was found to be 95% ± 4 at this ratio. LPHNP-IGF-1R siRNA complex could be able to down-regulate the target more efficiently when it compared with the corresponded controls (P < 0.001).
Conclusion
In conclusion, our results suggest that DDAB cationic lipid and mPEG-PCL copolymer hybrid nanoparticle may be a good candidate for efficient siRNA delivery.








Similar content being viewed by others
Abbreviations
- LPHNPs:
-
Lipid polymer hybrid nanoparticles
- PEG:
-
Poly(ethylene glycol)
- PCL:
-
Poly(ε-caprolactone)
- DDAB:
-
Dimethyldioctadecylammonium bromide
- IGF-1R:
-
Insulin-like growth factor type I
- siRNA:
-
Small interfering RNA
- FT-IR:
-
Fourier transform infrared spectroscopy
- 1H NMR:
-
Proton nuclear magnetic resonance spectroscopy
- AFM:
-
Atomic force microscopy
- DLS:
-
Dynamic light scattering
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- PDI:
-
Polydispersity index
- N:P ratio:
-
Nitrogen:Phosphate ratio
- PBS:
-
Phosphate-buffered saline
References
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494.
Cheng JC, Moore TB, Sakamoto KM. RNA interference and human disease. Mol Genet Metab. 2003;80:121–8.
Leung RK, Whittaker PA. RNA interference: from gene silencing to gene-specific therapeutics. Pharmacol Ther. 2005;107:222–39.
Drude I, Dombos V, Vauleon S, Muller S. Drugs made of RNA: development and application of engineered RNAs for gene therapy. Mini Rev Med Chem. 2007;7:912–31.
Chalbatani GM, Dana H, Gharagouzloo E, et al. Small interfering RNAs (siRNAs) in cancer therapy: a nano-based approach. Int J Nanomed. 2019;14:3111.
Singh A, Trivedi P, Jain NK. Advances in siRNA delivery in cancer therapy. Artif Cells Nanomed Biotechnol. 2018;46:274–83.
Lee J-M, Yoon T-J, Cho Y-S. Recent developments in nanoparticle-based siRNA delivery for cancer therapy. Biomed Res Int. 2013;2013:782041.
Van de Water FM, Boerman OC, Wouterse AC, Peters JG, Russel FG, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab Dispos. 2006;34:1393–7.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958.
Cho K, Wang X, Nie S, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–6.
Puri A, Loomis K, Smith B, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–80.
Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–9.
Taetz S, Bochot A, Surace C, et al. Hyaluronic acid-modified DOTAP/DOPE liposomes for the targeted delivery of anti-telomerase siRNA to CD44-expressing lung cancer cells. Oligonucleotides. 2009;19:103–16.
Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5:25–44.
Wu SY, McMillan NA. Lipidic systems for in vivo siRNA delivery. AAPS J. 2009;11:639–52.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751.
Singha K, Namgung R, Kim WJ. Polymers in small-interfering RNA delivery. Nucleic Acid Ther. 2011;21:133–47.
Hamidi M, Shahbazi MA, Rostamizadeh K. Copolymers: efficient carriers for intelligent nanoparticulate drug targeting and gene therapy. Macromol Biosci. 2012;12:144–64.
Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–28.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145.
Hadinoto K, Sundaresan A, Cheow WS. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85:427–43.
Farabaugh SM, Boone DN, Lee AV. Role of IGF1R in breast cancer subtypes, stemness, and lineage differentiation. Front Endocrinol. 2015;6:59.
Chan JY, LaPara K, Yee D. Disruption of insulin receptor function inhibits proliferation in endocrine-resistant breast cancer cells. Oncogene. 2016;35(32):4235–43.
Shu S, Yang Y, Li X, et al. Down-regulation of IGF-1R expression inhibits growth and enhances chemosensitivity of endometrial carcinoma in vitro. Mol Cell Biochem. 2011;353:225–33.
Yavari K, Taghikhani M, Maragheh MG, et al. SiRNA-mediated IGF-1R inhibition sensitizes human colon cancer SW480 cells to radiation. Acta Oncol. 2010;49:70–5.
Fathi M, Taghikhani M, Ghannadi-Maragheh M, Yavari K. Demonstration of dose dependent cytotoxic activity in SW480 colon cancer cells by 177 Lu-labeled siRNA targeting IGF-1R. Nucl Med Biol. 2013;40:529–36.
Fathi M, Yavari K, Taghikhani M, Maragheh MG. Synthesis of a stabilized 177Lu–siRNA complex and evaluation of its stability and RNAi activity. Nucl Med Commun. 2015;36:636–45.
Danafar H, Davaran S, Rostamizadeh K, Valizadeh H, Hamidi M. Biodegradable m-PEG/PCL core-shell micelles: preparation and characterization as a sustained release formulation for curcumin. Adv Pharm Bull. 2014;4:501.
Fang RH, Aryal S, Hu C-MJ, Zhang L. Quick synthesis of lipid− polymer hybrid nanoparticles with low polydispersity using a single-step sonication method. Langmuir. 2010;26:16958–62.
Filion MC, Phillips NC. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim Biophys Acta Biomembr. 1997;1329:345–56.
Li W, Szoka FC. Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–49.
Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–17.
Inglut CT, Sorrin AJ, Kuruppu T, et al. Immunological and toxicological considerations for the design of liposomes. Nanomaterials. 2020;10:190.
Yang X-Z, Dou S, Wang Y-C, et al. Single-step assembly of cationic lipid–polymer hybrid nanoparticles for systemic delivery of siRNA. ACS Nano. 2012;6:4955–65.
Zhang L, Chan JM, Gu FX, et al. Self-assembled lipid− polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2:1696–702.
Tan S, Li X, Guo Y, Zhang Z. Lipid-enveloped hybrid nanoparticles for drug delivery. Nanoscale. 2013;5:860–72.
Mandal B, Bhattacharjee H, Mittal N, et al. Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine. 2013;9:474–91.
Dehaini D, Fang RH, Luk BT, et al. Ultra-small lipid–polymer hybrid nanoparticles for tumor-penetrating drug delivery. Nanoscale. 2016;8:14411–9.
Wen J, Kim GJ, Leong KW. Poly (d, llactide–co-ethyl ethylene phosphate) s as new drug carriers. J Control Release. 2003;92:39–48.
Li Y, Huang X, Lee RJ, et al. Synthesis of polymer-lipid nanoparticles by microfluidic focusing for siRNA delivery. Molecules. 2016;21:1314.
Shi J, Xiao Z, Votruba AR, Vilos C, Farokhzad OC. Differentially charged hollow core/shell lipid–polymer–lipid hybrid nanoparticles for small interfering RNA delivery. Angew Chem Int Ed Engl. 2011;123:7165–9.
Hasan W, Chu K, Gullapalli A, et al. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2011;12:287–92.
Monirinasab H, Asadi H, Rostamizadeh K, Esmaeilzadeh A, Khodaei M, Fathi M. Novel lipid-polymer hybrid nanoparticles for siRNA delivery and IGF-1R gene silencing in breast cancer cells. J Drug Deliv Sci Technol. 2018;48:96–105.
Acknowledgements
The authors are hereby grateful to the deputy of research and technology, and Cancer Gene Therapy Research Center, Zanjan University of Medical Sciences, Zanjan, Iran.
Funding
This study was funded by a grant (No: A-12-802-7) and ethics number (ZUMS.REC.1394.08) from Department of Clinical Biochemistry, School of Medicine, Zanjan University of Medical Sciences (ZUMS), Zanjan, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was aprooved by ethical committee of Zanjan University of Medical Sciences with a number of ZUMS.REC.1394.08.
Informed consent
Informed consent was obtained from all individuals participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khodaei, M., Rostamizadeh, K., Taromchi, A.H. et al. DDAB cationic lipid-mPEG, PCL copolymer hybrid nano-carrier synthesis and application for delivery of siRNA targeting IGF-1R into breast cancer cells. Clin Transl Oncol 23, 1167–1178 (2021). https://doi.org/10.1007/s12094-020-02507-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02507-3