Abstract
Objective
To identify distinct trajectories of toxicity in colorectal cancer (CRC) patients after adjuvant chemotherapy and its impact on quality of life (QoL) and psychological symptoms.
Methods
A prospective, multicenter study was conducted in 157 patients. A latent class analysis defined the unobserved latent constructs that can be predicted as symptom clusters, considering the intensity of four types of adverse events (AEs). Patients completed EORTC-QLQ-C30, BSI-18, PDRQ-9, and DRS scales.
Results
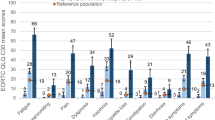
Ninety-six percent had some degree of toxicity, with grades 3–4 being the most common: neurotoxicity (7.2%), hematological (13.1%), digestive (5.2%), and skin toxicity (1.4%). Three distinct latent classes were identified (high [72.5%], mild [16.9%], and low [10.6%] toxicity). Patients with high toxicity had the worst QoL scores and moderately high somatization and psychological distress scores.
Conclusions
Adjuvant chemotherapy for CRC was associated with frequent toxicity that negatively impacted QoL and psychological wellbeing.

Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz AG, Greene FL. AJCC cancer staging manual. New York: Springer; 2010.
Weiser MR, Landmann RG, Kattan MW, Gonen M, Shia J, Chou J, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol. 2008;26:380–5.
Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–806.
André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–16.
Haller DG, Tabernero J, Maroun J, De Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–71.
Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Derogatis LR. BSI 18, Brief symptom inventory 18: administration, scoring and procedures manual. Incorporated: NCS Pearson; 2001.
Van der Feltz-Cornelis CM, Van Oppen P, Van Marwijk HWJ, De Beurs E, Van Dyck R. A patient-doctor relationship questionnaire (PDRQ-9) in primary care: development and psychometric evaluation. Gen Hosp Psychiatry. 2004;26:115–20.
Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a decision regret scale. Med Decis Mak. 2003;23:281–92.
Calderon C, Ferrando PJ, Lorenzo-Seva U, Higuera O, y Cajal TR, Rogado J, et al. Validity and reliability of the decision regret scale in cancer patients receiving adjuvant chemotherapy. J Pain Symptom Manage. 2019;57:828–34.
Collins LM, Lanza ST. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. 1st ed. Hoboken: John Wiley & Sons; 2010.
Hagenaars JA, McCutcheon AL. Applied latent class analysis. Cambridge: Cambridge University Press; 2002.
Shah MA, Renfro LA, Allegra CJ, André T, De Gramont A, Schmoll H-J, et al. Impact of patient factors on recurrence risk and time dependency of oxaliplatin benefit in patients with colon cancer: analysis from modern-era adjuvant studies in the adjuvant colon cancer end points (ACCENT) database. J Clin Oncol. 2016;34:843.
García-García T, Carmona-Bayonas A, Jimenez-Fonseca P, Jara C, Beato C, Castelo B, et al. Biopsychosocial and clinical characteristics in patients with resected breast and colon cancer at the beginning and end of adjuvant treatment. BMC Cancer. 2019;19:1143.
Acknowledgements
We thank Priscilla Chase Duran for editing the manuscript; Natalia Cateriano, Miguel Vaquero, and IRICOM S.A. for supporting the registry website
Funding
This study was supported by the FSEOM-Onvida for Projects on Long Survivors and Quality of Life. SEOM (Spanish Society of Medical Oncology) 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest. This is an academic study.
Ethical approval
This study was performed in accordance with the ethical standards of the Declaration of Helsinki and its later amendments. It is an observational, non-interventional trial.
Informed consent
Signed informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Annexed Table: Participating centers
Annexed Table: Participating centers
-
1.
Hospital Universitario de Canarias, Tenerife
-
2.
Hospital Universitario Central de Asturias, Oviedo
-
3.
Hospital Universitario La Paz, Madrid
-
4.
Hospital Universitario Morales Meseguer, Murcia
-
5.
Hospital Universitario Fundación Alcorcon, Madrid
-
6.
Hospital Universitario Marqués de Valdecilla, Santander
-
7.
Hospital Universitario Virgen de La Luz, Cuenca
-
8.
Grupo Hospitalario Quirón, Sevilla
-
9.
Hospital Universitario Son Espases, Mallorca
-
10.
Hospital Universitario Santa Creu i Sant Pau, Barcelona
-
11.
Hospital Universitario Insular de Gran Canaria, Las Palmas
-
12.
Hospital Universitario La Princesa, Madrid
Rights and permissions
About this article
Cite this article
Gomez, D., Calderón, C., Carmona-Bayonas, A. et al. Impact of adjuvant therapy toxicity on quality of life and emotional symptoms in patients with colon cancer: a latent class analysis. Clin Transl Oncol 23, 657–662 (2021). https://doi.org/10.1007/s12094-020-02454-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02454-z