Abstract
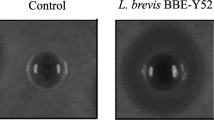
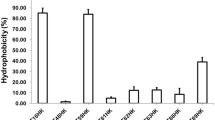
Members of the lactic acid bacillus group are well-known probiotics and primarily isolated from fermented food, dairy products, intestinal and gut environment of human. Since probiotics from the human source are preferred, there exists a huge repertoire of lactobacilli in the human oral cavity which could prove a much better niche to be exploited for these beneficial microorganisms. Therefore, in this study, four lactobacilli strains, including strain DISK7, reported earlier, isolated from dental plaque samples of a healthy humans were evaluated for their probiotic potential. Strains displayed 99.9% of 16S rRNA gene sequence identity with species of the genera Lactobacillus and Limosilactobacillus. All strains showed lactic acid production, tolerance to low pH and antibiotic sensitivity. Variations were observed among strains in their aggregation ability, biofilm formation, bile salt resistance and cholesterol degradation. Further, we analyzed the interaction of strains with other oral commensals and opportunistic pathogens in co-culture experiments. Isolates DISK7 and DISK26 exhibited high co-aggregation (> 70%) with secondary colonizers, Streptococcus pyogenes and Veillonella parvula, respectively, but their aggregation ability was decreased with opportunistic pathogens. Furthermore, strains showed a substantial increase in biofilm in co-culture with other Lactobacillus isolates, indicating their ability to proliferate commensal bacteria in the oral environment. These microbes continually evolve in terms of niche adaptation as evidenced in genome analysis. The highlight of the investigation is the isolation and evaluation of the probiotic lactobacilli from the human oral cavity, which could prove a much better niche to be exploited for the effective commercialization of these beneficial microbes. Taken together, probiotic properties and interaction with commensal bacteria, these isolates exhibit the huge potential to be developed as alternative bioresource agents for maintenance of oral health.





Similar content being viewed by others
Data Availability
16S rRNA gene was submitted to EMBL with accession Nos. for DISK12, DISK16 and DISK26 were OP728783, OP729196 and OP729175, respectively. Whole genome sequences of all strains were submitted to NCBI and available with accession numbers Accession nos., DISK12, MKXG01000001.1; DISK16, MJER01000001.1; DISK26, MJES01000001.1.
References
Tsuda H, Miyamoto T (2010) Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food, 2002. Food Sci Technol Res 16:87–92
Pereira DI, Gibson GR (2002) Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl Environ Microbiol 68:4689–4693. https://doi.org/10.1128/AEM.68.9.4689-4693.2002
Singh SS, Akhtar M, Sharma D, Mandal SM, Korpole S (2021) Characterization of iturin V, a novel antimicrobial lipopeptide from a potential probiotic strain Lactobacillus sp. M31. Probiot Antimicrob Prot 13:1766–1779. https://doi.org/10.1007/s12602-021-09796-2
Hudault S, Liévin V, Bernet-Camard MF, Servin AL (1997) Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl Environ Microbiol 63:513–518. https://doi.org/10.1128/aem.63.2.513-518.1997
Pino A, Bartolo E, Caggia C, Cianci A, Randazzo CL (2019) Detection of vaginal lactobacilli as probiotic candidates. Sci Rep 9:1. https://doi.org/10.1038/s41598-019-40304-3
Sornplang P, Piyadeatsoontorn S (2016) Probiotic isolates from unconventional sources: a review. J Anim Sci Technol 58:1–1. https://doi.org/10.1186/s40781-016-0108-2
Zheng J, Wittouck S, Salvetti E, Franz CM, Harris HM, Mattarelli P, Otoole PW, Pot B, Vandamme P, Walter J, Watanabe K (2020) A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70:2782–2858. https://doi.org/10.1099/ijsem.0.004107
Walter J (2008) Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol 74:4985–4996. https://doi.org/10.1128/AEM.00753-08
Caufield PW, Schön CN, Saraithong P, Li Y, Argimón S (2015) Oral lactobacilli and dental caries: a model for niche adaptation in humans. J Dent Res 94:110S-S118. https://doi.org/10.1177/0022034515576052
Kim JA, Bayo J, Cha J, Choi YJ, Jung MY, Kim DH, Kim Y (2019) Investigating the probiotic characteristics of four microbial strains with potential application in feed industry. PLoS ONE 14:e0218922. https://doi.org/10.1371/journal.pone.0218922
Urvashi SD, Sharma S, Pal V, Lal R, Patil P, Grover V, Korpole S (2020) Bacterial populations in subgingival plaque under healthy and diseased conditions: genomic insights into oral adaptation strategies by Lactobacillus sp. strain DISK7. Indian J Microbiol 60:78–86. https://doi.org/10.1007/s12088-019-00828-8
Nallabelli N, Patil PP, Pal VK, Singh N, Jain A, Patil PB, Korpole GV, S, (2016) Biochemical and genome sequence analyses of Megasphaera sp. strain DISK18 from dental plaque of a healthy individual reveal’s commensal lifestyle. Sci Rep 6:1–3. https://doi.org/10.1038/srep33665
Richter M, Rosselló-Móra R (2009) Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci 106:19126–19131. https://doi.org/10.1073/pnas.0906412106
Malik A, Sakamoto M, Hanazaki S, Osawa M, Suzuki T, Tochigi M, Kakii K (2003) Coaggregation among nonflocculating bacteria isolated from activated sludge. Appl Environ Microbiol 69:6056–6063. https://doi.org/10.1128/AEM.69.10.6056-6063.2003
Kumar R, Grover S, Batish VK (2012) Bile salt hydrolase (Bsh) activity screening of Lactobacilli: in vitro selection of indigenous Lactobacillus strains with potential bile salt hydrolysing and cholesterol-lowering ability. Probiot Antimicrob Prot 4:162–172. https://doi.org/10.1007/s12602-012-9101-3
Gilliland SE, Nelson CR, Maxwell C (1985) Assimilation of cholesterol by Lactobacillus acidophilus. Appl Environ Microbiol 49:377–381. https://doi.org/10.1128/aem.49.2.377-381.1985
Koul S, Kalia VC (2016) Comparative genomics reveals biomarkers to identify Lactobacillus species. Indian J Microbiol 56:265–276. https://doi.org/10.1007/s12088-016-0605-5
Kalia VC, Kumar R, Kumar P, Koul S (2016) A genome-wide profiling strategy as an aid for searching unique identification biomarkers for Streptococcus. Indian J Microbiol 56:46–58. https://doi.org/10.1007/s12088-015-0561-5
Tanizawa Y, Tada I, Kobayashi H, Endo A, Maeno S, Toyoda A, Arita M, Nakamura Y, Sakamoto M, Ohkuma M, Tohno M (2018) Lactobacillus paragasseri sp. nov., a sister taxon of Lactobacillus gasseri, based on whole-genome sequence analyses. Int J Syst Evol Microbiol 68:3512–3517. https://doi.org/10.1099/ijsem.0.003020
Cornejo OE, Lefébure T, Bitar PD, Lang P, Richards VP, Eilertson K, Do T, Beighton D, Zeng L, Ahn SJ, Burne RA, Siepel A, Bustamante CD, Stanhope MJ (2013) Evolutionary and population genomics of the cavity causing bacteria Streptococcus mutans. Mol Biol Evol 30:881–93. https://doi.org/10.1093/molbev/mss278
Kalia VC, Raju SC, Purohit HJ (2011) Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone-acylase and -lactonase. Open Microbiol J 5:1–13. https://doi.org/10.2174/1874285801105010001
Colombo AP, do Souto RM, da Silva-Boghossian CM, Miranda R, Lourenço TG (2015) Microbiology of oral biofilm-dependent diseases: have we made significant progress to understand and treat these diseases? Curr Oral Health Rep 2:37–47. https://doi.org/10.1007/s40496-014-0041-8
Mäkeläinen H, Hasselwander O, Rautonen N, Ouwehand AC (200) Panose, a new prebiotic candidate. Lett Appl Microbiol 49:666–672
Ventura M, Turroni F, Zomer A, Foroni E, Giubellini V, Bottacini F, Canchaya C, Claesson MJ, He F, Mantzourani M, Mulas L (2009) The Bifidobacterium dentium Bd1 genome sequence reflects its genetic adaptation to the human oral cavity. PLoS Genet 5:e1000785. https://doi.org/10.1371/journal.pgen.1000785
Mendes-Soares H, Suzuki H, Hickey RJ, Forney LJ (2014) Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J Bacteriol 196:1458–1470. https://doi.org/10.1128/JB.01439-13
Broadbent JR, Neeno-Eckwall EC, Stahl B, Tandee K, Cai H, Morovic W, Horvath P, Heidenreich J, Perna NT, Barrangou R, Steele JL (2012) Analysis of the Lactobacillus casei supragenome and its influence in species evolution and lifestyle adaptation. BMC Genom 13:1–8. https://doi.org/10.1186/1471-2164-13-533
Sabir F, Beyatli Y, Cokmus C, Onal-Darilmaz D (2010) Assessment of potential probiotic properties of Lactobacillus spp., Lactococcus spp., and Pediococcus spp. strains isolated from kefir. J Food Sci 75:M568–M573. https://doi.org/10.1111/j.1750-3841.2010.01855.x
Yadav AS, Kolluri G, Gopi M, Karthik K, Malik Y, Dharma K (2016) Exploring alternatives to antibiotics as health promoting agents in poultry-a review. J Exp Biol 4:368–383. https://doi.org/10.18006/2016.4(3S).368.383
Zhu L, Kreth J (2012) The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid Med Cell Longev 2012:717843. https://doi.org/10.1155/2012/717843
Ferreira CL, Grześkowiak Ł, Collado MC, Salminen S (2011) In vitro evaluation of Lactobacillus gasseri strains of infant origin on adhesion and aggregation of specific pathogens. J Food Prot 9:1482–7. https://doi.org/10.4315/0362-028X.JFP-11-074
Ocaña VS, Nader-Macías ME (2002) Vaginal lactobacilli: self-and co-aggregating ability. Br J Biomed Sci 59:183–190. https://doi.org/10.1080/09674845.2002.11783657
Kolenbrander PE, Andersen RN, Holdeman LV (1985) Co-aggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun 48:741–746. https://doi.org/10.1128/iai.48.3.741-746.1985
Takahashi N (2015) Oral microbiome metabolism: From “who are they?” to “what are they doing?” J Dent Res 94:1628–1637. https://doi.org/10.1177/0022034515606045
Kalia VC, Wood TK, Kumar P (2014) Evolution of resistance to quorum-sensing inhibitors. Microbial Ecol 68:13–23. https://doi.org/10.1007/s00248-013-0316-y
Angelin J, Kavitha M (2020) Exopolysaccharides from probiotic bacteria and their health potential. Int J Biol Macromol 162:853–865. https://doi.org/10.1016/j.ijbiomac.2020.06.190
Koul S, Prakash J, Mishra A, Kalia VC (2016) Potential emergence of multi-quorum sensing inhibitor resistant (MQSIR) bacteria. Ind J Microbiol 56:1–8. https://doi.org/10.1007/s12088-015-0558-0
Aoudia N, Rieu A, Briandet R, Deschamps J, Chluba J, Jego G, Garrido C, Guzzo J (2016) Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: Effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol 53:51–59. https://doi.org/10.1016/j.fm.2015.04.009
Sharma P, Tomar SK, Sangwan V, Goswami P, Singh R (2016) Antibiotic resistance of Lactobacillus sp. isolated from commercial probiotic preparations. J Food Saf 36:38–51. https://doi.org/10.1111/jfs.12211
Jose NM, Bunt CR, Hussain MA (2015) Implications of antibiotic resistance in probiotics. Food Rev Int 31:52–62. https://doi.org/10.1080/87559129.2014.961075
Acknowledgements
The financial research support grant was issued by SERB-DST, Department of Science and Technology and Council of Scientific and Industrial Research, Govt of India. CSIR fellowship to Stanzin Choksket and Harshvardhan is duly acknowledged.
Author information
Authors and Affiliations
Contributions
SC, SS, SK and VG were involved in conceptualization. SC, SS, HV, VP, AJ, SK and VG were involved in designing and performing experiments. SS and PBP were involved in the genome sequencing. SC, SS, PBP, SK and VG were involved in data analysis. SC, SS, SK and VG were involved in writing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choksket, S., Sharma, S., Harshvardhan et al. Evaluation of Human Dental Plaque Lactic Acid Bacilli for Probiotic Potential and Functional Analysis in Relevance to Oral Health. Indian J Microbiol 63, 520–532 (2023). https://doi.org/10.1007/s12088-023-01108-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-023-01108-2