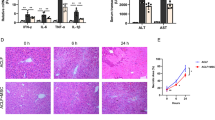
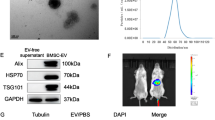
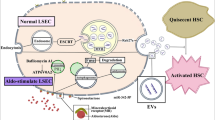
Abstract
Background
Impaired liver regeneration in hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) patients is closely related to prognosis; however, the mechanisms are not yet defined. Liver-derived extracellular vesicles (EVs) may be involved in the dysregulation of liver regeneration. Clarifying the underlying mechanisms will improve the treatments for HBV-ACLF.
Methods
EVs were isolated by ultracentrifugation from liver tissues of HBV-ACLF patients (ACLF_EVs) after liver transplantation, and their function was investigated in acute liver injury (ALI) mice and AML12 cells. Differentially expressed miRNAs (DE-miRNAs) were screened by deep miRNA sequencing. The lipid nanoparticle (LNP) system was applied as a carrier for the targeted delivery of miRNA inhibitors to improve its effect on liver regeneration.
Results
ACLF_EVs inhibited hepatocyte proliferation and liver regeneration, with a critical role of miR-218-5p. Mechanistically, ACLF_EVs fused directly with target hepatocytes and transferred miR-218-5p into hepatocytes, acting by suppressing FGFR2 mRNA and inhibiting the activation of ERK1/2 signaling pathway. Reducing the level of miR-218-5p expression in the liver of ACLF mice partially restored liver regeneration ability.
Conclusion
The current data reveal the mechanism underlying impaired liver regeneration in HBV-ACLF that promotes the discovery of new therapeutic approaches.
Graphical abstract








Similar content being viewed by others
Data availability
Sequencing data generated in this study has been deposited at the National Omics Data Encyclopedia (NODE) under the accession code OEP004077 (https://www.biosino.org/node/project/detail/OEP004077).
Abbreviations
- CCl4 :
-
Carbon tetrachloride
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- LDH:
-
Lactate dehydrogenase
- TBIL:
-
Total bilirubin
- BUN:
-
Blood urea nitrogen
- ACLF:
-
Acute-on-chronic liver failure
- HC:
-
Healthy control
- CI:
-
Chronic inflammation
- EVs:
-
Extracellular vesicles
- ACLF_EVs:
-
Liver-derived extracellular vesicles from ACLF
- HC_EVs:
-
Liver-derived extracellular vesicles from healthy control
- H&E staining:
-
Hematoxylin–eosin staining
- PCNA:
-
Proliferating cell nuclear antigen
- CCK-8:
-
Cell Counting Kit-8
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- EdU:
-
5-Ethynyl-2′-deoxyuridine
- LC:
-
Liver cirrhosis
- LC_EVs:
-
Liver-derived extracellular vesicles from LC
- DE-miRNAs:
-
Differentially expressed miRNAs
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GO:
-
Gene ontology
- FGFR2:
-
Fibroblast Growth Factor Receptor 2
- ALB:
-
Albumin
- miR_veh:
-
miR-vehicle
- miR_inh:
-
miR-inhibitor
- miR_ago:
-
miR-agomir
- miR_ant:
-
miR-antagomir
- LNP:
-
Lipid nanoparticle
- NPCs:
-
Nonparenchymal cells
References
Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 2018;67:2181–2191
Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med 2020;382:2137–2145
Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol 2016;13:131–149
Belli LS, Duvoux C, Artzner T, Bernal W, Conti S, Cortesi PA, et al. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: results of the ELITA/EF-CLIF collaborative study (ECLIS). J Hepatol 2021;75:610–622
Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology (Baltim, MD) 2017;65:1384–1392
Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology (Baltim, MD) 2004;39:1477–1487
Xiang X, Feng D, Hwang S, Ren T, Wang X, Trojnar E, et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J Hepatol 2020;72:736–745
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018;19:213–228
Luo C, Xin H, Zhou Z, Hu Z, Sun R, Yao N, et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology (Baltim, MD) 2022;76:982–999
Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol 2016;64:60–68
Tan CY, Lai RC, Wong W, Dan YY, Lim S-K, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther 2014;5:76
Kakizaki M, Yamamoto Y, Nakayama S, Kameda K, Nagashima E, Ito M, et al. Human hepatocyte-derived extracellular vesicles attenuate the carbon tetrachloride-induced acute liver injury in mice. Cell Death Dis 2021;12:1010
Wang J, Li L, Zhang Z, Zhang X, Zhu Y, Zhang C, et al. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab 2022;34(1264–1279): e8
Lee J, Kim SR, Lee C, Jun YI, Bae S, Yoon YJ, et al. Extracellular vesicles from in vivo liver tissue accelerate recovery of liver necrosis induced by carbon tetrachloride. J Extracell Vesicles 2021;10: e12133
Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol 2016;65:213–221
Wang X, He Y, Mackowiak B, Gao B. MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 2021;70:784–795
Ng R, Song G, Roll GR, Frandsen NM, Willenbring H. A microRNA-21 surge facilitates rapid cyclin D1 translation and cell cycle progression in mouse liver regeneration. J Clin Investig 2012;122:1097–1108
Chang YM, Chen PC, Hsu CP, Ma PF, Chen HL, Hsu SH. Loss of hepatic miR-194 promotes liver regeneration and protects from acetaminophen-induced acute liver injury. Biochem Pharmacol 2022;195: 114862
Wen Y, Peng SF, Fu L, Fu XY, Wu DX, Liu BJ, et al. Serum levels of miRNA in patients with hepatitis B virus-associated acute-on-chronic liver failure. Hepatobiliary Pancreat Dis Int 2018;17:126–132
Tao YC, Wang ML, Wang M, Ma YJ, Bai L, Feng P, et al. Quantification of circulating miR-125b-5p predicts survival in chronic hepatitis B patients with acute-on-chronic liver failure. Dig Liver Dis 2019;51:412–418
Crescitelli R, Lasser C, Lotvall J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat Protoc 2021;16:1548–1580
Xu D, Chen L, Chen X, Wen Y, Yu C, Yao J, et al. The triterpenoid CDDO-imidazolide ameliorates mouse liver ischemia-reperfusion injury through activating the Nrf2/HO-1 pathway enhanced autophagy. Cell Death Dis 2017;8:e2983
Ray AT, Mazot P, Brewer JR, Catela C, Dinsmore CJ, Soriano P. FGF signaling regulates development by processes beyond canonical pathways. Genes Dev 2020;34:1735–1752
Tian ZJ, An W. ERK1/2 contributes negative regulation to STAT3 activity in HSS-transfected HepG2 cells. Cell Res 2004;14:141–147
Yang T, Poenisch M, Khanal R, Hu Q, Dai Z, Li R, et al. Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J Hepatol 2021;75:1420–1433
Gokita K, Inoue J, Ishihara H, Kojima K, Inazawa J. Therapeutic potential of LNP-mediated delivery of miR-634 for cancer therapy. Mol Ther Nucleic Acids 2020;19:330–338
Engelmann C, Habtesion A, Hassan M, Kerbert AJ, Hammerich L, Novelli S, et al. Combination of G-CSF and a TLR4 inhibitor reduce inflammation and promote regeneration in a mouse model of ACLF. J Hepatol 2022;77:1325–1338
Deng M, Zeng C, Lu X, He X, Zhang R, Qiu Q, et al. miR-218 suppresses gastric cancer cell cycle progression through the CDK6/Cyclin D1/E2F1 axis in a feedback loop. Cancer Lett 2017;403:175–185
Yu J, Yang M, Zhou B, Luo J, Zhang Z, Zhang W, et al. CircRNA-104718 acts as competing endogenous RNA and promotes hepatocellular carcinoma progression through microRNA-218-5p/TXNDC5 signaling pathway. Clin Sci (Lond) 2019;133:1487–1503
Acknowledgements
The authors acknowledge the patients, study investigators, and coordinators for their contributions in this study.
Funding
This study was supported by the National Natural Science Foundation of China (82170646 to Hualian Hang), “Key Project” of Shanghai Jiao Tong University Medical-Industry Intersection (YG2021ZD10 to Hualian Hang), Clinical Research Innovation Cultivation Fund of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (RJPY-DZX-006 to Hualian Hang).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. SZ, JY and KR designed research; SZ, JY and KR and JC performed research; LM, YY, ZL and ZZ carried data analysis; YQ, MC and HH supervised research; SZ, JY and KR wrote the paper. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Senquan Zhang, Jie Yu, Keqiang Rao, Jie Cao, Lijie Ma, Yeping Yu, Zhe Li, Zhaokai Zeng, Yongbing Qian, Mo Chen, Hualian Hang have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Ethics Committee of Renji Hospital of Shanghai Jiao Tong University School of Medicine, China) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Yu, J., Rao, K. et al. Liver-derived extracellular vesicles from patients with hepatitis B virus-related acute-on-chronic liver failure impair hepatic regeneration by inhibiting on FGFR2 signaling via miR-218-5p. Hepatol Int 17, 833–849 (2023). https://doi.org/10.1007/s12072-023-10513-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10513-0