Abstract
Background
Most patients with autosomal-dominant polycystic kidney disease (ADPKD) develop liver cysts and polycystic liver disease as they age. To date, no simple clinical indicator has been confirmed to predict polycystic liver disease exacerbation. Furthermore, the effect of the type and location of mutation on disease progression of polycystic liver disease remains unclear. Here, we aimed to establish a simple liver cyst indicator for clinical practice and investigate whether gene mutations determined liver phenotype in patients with autosomal-dominant polycystic kidney disease.
Methods
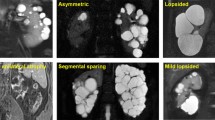
In total, 129 patients with ADPKD were enrolled and liver cyst indicators were assessed based on mutation type (truncating mutation: nonsense, frameshift, and splicing mutation; non-truncating mutation: substitution) and mutation position. Liver cyst severity was determined using Gigot and Drenth classifications, based on their number, maximum diameter, and area ratio with the liver.
Results
We observed an overall prevalence of 62.8% for polycystic liver disease. Patients with PKD1 nonsense mutations, a type of PKD1 truncating mutation, exhibited more severe liver disease phenotypes than those without the mutation. We identified maximum diameter as a potential liver cyst indicator. Moreover, a subgroup analysis that included a PKD1 nonsense mutation cohort revealed that genetic mutations located closer to the 5ʹ end of PKD1 were associated with a maximum diameter index value ≥ 6 cm.
Conclusion
PKD1 nonsense mutations were associated with liver cyst severity, which along with maximum diameter index as a simple clinical indicator for liver cysts, may improve the treatment of polycystic liver disease associated with ADPKD.


Similar content being viewed by others
References
van Aerts RMM, van de Laarschot LFM, Banales JM, Drenth JPH. Clinical management of polycystic liver disease. J Hepatol 2018;68:827–837
Mochizuki T, Tsuchiya K, Nitta K. Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol 2013;17:317–326
Masyuk TV, Masyuk AI, LaRusso NF. Polycystic liver disease: the interplay of genes causative for hepatic and renal cystogenesis. Hepatology 2018;67:2462–2464
Zhang ZY, Wang ZM, Huang Y. Polycystic liver disease: classification, diagnosis, treatment process, and clinical management. World J Hepatol 2020;12:72–83
Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 1996;272:1339–1342
Fedeles SV, Tian X, Gallagher AR, Mitobe M, Nishio S, Lee SH, Cai Y, Geng L, Crews CM, Somlo S. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet 2011;43:639–647
Lantinga MA, Gevers TJ, Drenth JP. Evaluation of hepatic cystic lesions. World J Gastroenterol. 2013;19:3543–54.
Abu-Wasel B, Walsh C, Keough V, Molinari M. Pathophysiology, epidemiology, classification and treatment options for polycystic liver diseases. World J Gastroenterol 2013;19:5775–5786
Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology 1990;11:1033–1037
Kataoka H, Ono K, Mochizuki T, Hanafusa N, Imai E, Hishida A, Nitta K. A Body mass index-based cross-classification approach for the assessment of prognostic factors in chronic kidney disease progression. Kidney Blood Press Res 2019;44:362–383
Cornec-Le Gall E, Torres VE, Harris PC. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol 2018;29:13–23
Chebib FT, Jung Y, Heyer CM, Irazabal MV, Hogan MC, Harris PC, Torres VE, El-Zoghby ZM. Effect of genotype on the severity and volume progression of polycystic liver disease in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2016;31:952–960
Kataoka H, Fukuoka H, Makabe S, Yoshida R, Teraoka A, Ushio Y, Akihisa T, Manabe S, Sato M, Mitobe M, Tsuchiya K, Nitta K, Mochizuki T. Prediction of renal prognosis in patients with autosomal dominant polycystic kidney disease using pkd1/pkd2 mutations. J Clin Med 2020;9:146
Mochizuki T, Teraoka A, Akagawa H, Makabe S, Akihisa T, Sato M, Kataoka H, Mitobe M, Furukawa T, Tsuchiya K, Nitta K. Mutation analyses by next-generation sequencing and multiplex ligation-dependent probe amplification in Japanese autosomal dominant polycystic kidney disease patients. Clin Exp Nephrol 2019;23:1022–1030
Drenth JP, Chrispijn M, Nagorney DM, Kamath PS, Torres VE. Medical and surgical treatment options for polycystic liver disease. Hepatology 2010;52:2223–2230
Muto S, Ando M, Nishio S, Hanaoka K, Ubara Y, Narita I, Kamura K, Mochizuki T, Tsuchiya K, Tsuruya K, Horie S. The relationship between liver cyst volume and QOL in Japanese ADPKD patients. Clin Exp Nephrol 2020;24:314–322
Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710–718
Hoevenaren IA, Wester R, Schrier RW, McFann K, Doctor RB, Drenth JP, Everson GT. Polycystic liver: clinical characteristics of patients with isolated polycystic liver disease compared with patients with polycystic liver and autosomal dominant polycystic kidney disease. Liver Int 2008;28:264–270
Gigot JF, Jadoul P, Que F, Van Beers BE, Etienne J, Horsmans Y, Collard A, Geubel A, Pringot J, Kestens PJ. Adult polycystic liver disease: is fenestration the most adequate operation for long-term management? Ann Surg 1997;225:286–294
Torres VE, Chapman AB, Perrone RD, Bae KT, Abebe KZ, Bost JE, Miskulin DC, Steinman TI, Braun WE, Winklhofer FT, Hogan MC, Oskoui FR, Kelleher C, Masoumi A, Glockner J, Halin NJ, Martin DR, Remer E, Patel N, Pedrosa I, Wetzel LH, Thompson PA, Miller JP, Meyers CM, Schrier RW. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int 2012;81:577–585
Hogan MC, Abebe K, Torres VE, Chapman AB, Bae KT, Tao C, Sun H, Perrone RD, Steinman TI, Braun W, Winklhofer FT, Miskulin DC, Rahbari-Oskoui F, Brosnahan G, Masoumi A, Karpov IO, Spillane S, Flessner M, Moore CG, Schrier RW. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol 2015;13(155–164):e156
Kataoka H, Ohara M, Honda K, Mochizuki T, Nitta K. Maximal glomerular diameter as a 10-year prognostic indicator for IgA nephropathy. Nephrol Dial Transplant 2011;26:3937–3943
Cornec-Le Gall E, Audrezet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Ferec C, Le Meur Y. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 2013;24:1006–1013
Rossetti S, Burton S, Strmecki L, Pond GR, San Millan JL, Zerres K, Barratt TM, Ozen S, Torres VE, Bergstralh EJ, Winearls CG, Harris PC. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol 2002;13:1230–1237
Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 2012;122:4257–4273
Masyuk AI, Gradilone SA, LaRusso NF. Calcium signaling in cilia and ciliary-mediated intracellular calcium signaling: are they independent or coordinated molecular events? Hepatology 2014;60:1783–1785.
Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 2006;131:911–920
Alvaro D, Onori P, Alpini G, Franchitto A, Jefferson DM, Torrice A, Cardinale V, Stefanelli F, Mancino MG, Strazzabosco M, Angelico M, Attili A, Gaudio E. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am J Pathol 2008;172:321–332
Lu W, Shen X, Pavlova A, Lakkis M, Ward CJ, Pritchard L, Harris PC, Genest DR, Perez-Atayde AR, Zhou J. Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects. Hum Mol Genet 2001;10:2385–2396
Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Polycystic kidney disease. Nat Rev Dis Primers 2018;4:50
Funding
This study was supported in part by Japan Society for the Promotion of Science (KAKENHI Grant Number JP 15K09279) and by a Grant-in-Aid for Intractable Renal Diseases Research, Research on rare and intractable diseases, Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Contributions
Conceptualization: HK, and TM; data curation: SW, HK, NI, RY, and TM; formal analysis: HK; writing—original draft: HK; validation: HK, MS, SM (Shun Manabe), SM (Shiho Makabe), TA, YU, and TM; supervision: TM, KT, and KN. All authors contributed important intellectual content during manuscript drafting or revision and accept personal accountability for their contributions, and agree to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors had access to the study data and reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Toshio Mochizuki and Ken Tsuchiya received travel fees and honoraria for lectures from Otsuka Pharmaceutical Co. Toshio Mochizuki and Hiroshi Kataoka belong to an endowed department sponsored by Otsuka Pharmaceutical Co., Chugai Pharmaceutical Co., Kyowa Hakko Kirin Co., and JMS Co.
Ethics approval
The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2008 and approved by the Research Ethics Committee of Tokyo Women’s Medical University (No. 196B).
Animal Research (Ethics)
Not applicable.
Clinical Trials Registration
Not applicable.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Consent to participate
Informed consent was obtained from all patients to participate in the study.
Consent for publication
Informed consent was obtained from all patients to be included in this manuscript.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kataoka, H., Watanabe, S., Sato, M. et al. Predicting liver cyst severity by mutations in patients with autosomal-dominant polycystic kidney disease. Hepatol Int 15, 791–803 (2021). https://doi.org/10.1007/s12072-021-10176-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10176-9